Did your doctor get human testing initiated after this research came out? NO? Then you don't have a functioning stroke doctor. Run away.
Caffeic acid improves locomotor activity and lessens inflammatory burden in a mouse model of rotenone-induced nigral neurodegeneration: Relevance to Parkinson’s disease therapy August 2018
The latest here: Will your doctors redeem themselves by getting human testing done with this research?
Caffeic Acid, a Polyphenolic Micronutrient Rescues Mice Brains against Aβ-Induced Neurodegeneration and Memory Impairment
1
Division of Life Science and Applied Life
Science (BK21 FOUR), College of Natural Sciences, Gyeongsang National
University, Jinju 52828, Republic of Korea
2
Department of Psychiatry and
Neuropsychology, School for Mental Health and Neuroscience (MHeNs),
Maastricht University, 6229ER Maastricht, The Netherlands
3
Alz-Dementia Korea Co., Jinju 52828, Republic of Korea
*
Author to whom correspondence should be addressed.
†
These authors contributed equally to this work.
Antioxidants 2023, 12(6), 1284; https://doi.org/10.3390/antiox12061284
Received: 17 May 2023
/
Revised: 8 June 2023
/
Accepted: 12 June 2023
/
Published: 15 June 2023
(This article belongs to the Special Issue Oxidative Stress and Inflammation as Targets for Novel Preventive and Therapeutic Approaches in Non-communicable Diseases III)
Abstract
Oxidative stress plays an important role in
cognitive dysfunctions and is seen in neurodegeneration and Alzheimer’s
disease (AD). It has been reported that the polyphenolic compound
caffeic acid possesses strong neuroprotective and antioxidant effects.
The current study was conducted to investigate the therapeutic potential
of caffeic acid against amyloid beta (Aβ1–42)-induced oxidative stress and memory impairments. Aβ1–42
(5 μL/5 min/mouse) was administered intracerebroventricularly (ICV)
into wild-type adult mice to induce AD-like pathological changes.
Caffeic acid was administered orally at 50 mg/kg/day for two weeks to AD
mice. Y-maze and Morris water maze (MWM) behavior tests were conducted
to assess memory and cognitive abilities. Western blot and
immunofluorescence analyses were used for the biochemical analyses. The
behavioral results indicated that caffeic acid administration improved
spatial learning, memory, and cognitive abilities in AD mice. Reactive
oxygen species (ROS) and lipid peroxidation (LPO) assays were performed
and showed that the levels of ROS and LPO were markedly reduced in the
caffeic acid-treated mice, as compared to Aβ-induced AD mice brains.
Moreover, the expression of nuclear factor erythroid 2–related factor 2
(Nrf2) and heme oxygenase-1 (HO-1) were regulated with the
administration of caffeic acid, compared to the Aβ-injected mice. Next,
we checked the expression of ionized calcium-binding adaptor molecule 1
(Iba-1), glial fibrillary acidic proteins (GFAP), and other inflammatory
markers in the experimental mice, which suggested enhanced expression
of these markers in AD mice brains, and were reduced with caffeic acid
treatment. Furthermore, caffeic acid enhanced synaptic markers in the AD
mice model. Additionally, caffeic acid treatment also decreased Aβ and
BACE-1 expression in the Aβ-induced AD mice model.
1. Introduction
Alzheimer’s disease (AD) is the most prevalent cause of dementia, which impairs memory and cognitive functions [1].
AD is characterized by two main pathological hallmarks: the
accumulation of amyloid-beta (Aβ) peptide and the formation of
neurofibrillary tangles (NFTs) in the brain [2].
The main enzyme responsible for the production of Aβ is β-secretase,
also known as β-site amyloid precursor protein-cleaving enzyme-1
(BACE-1), which generates toxic Aβ peptide that causes AD-associated
pathological changes and neurodegeneration [3,4].
Neurofibrillary tangles are formed from the hyperphosphorylation of
tau, which is a microtubule-associated protein that plays a major role
in the stabilization of neuronal microtubules and provides the track for
intracellular transport. In the hyperphosphorylated state, the tau
loses its capacity to bind to the microtubules and is unable to maintain
the cytoskeleton organization, resulting in misfolded proteins and NFTs
[5,6].
The accumulation of these two pathological proteins in the brain leads
to oxidative stress, neuroinflammation, downregulation of the brain’s
neurotrophic factors, and synaptic dysfunction [7].
Accumulation of Aβ in the brain triggers reactive oxygen species (ROS)
formation and lipid peroxidation, which disrupt the intracellular
defense mechanisms. The endogenous cellular antioxidant mechanism is
solely regulated by certain factors, such as nuclear factor erythroid
2–related factor 2 (Nrf2) and its associated genes. Nrf2 is a major
signaling pathway responsible for regulating reactive oxygen and redox
signaling by activating the phase II detoxification enzymes [8,9].
Nrf2 acts as a modulator of cellular antioxidant and detoxification
defense mechanisms, and its activation can reduce cellular injury and
insult in several organs and tissues. Several studies have suggested
that boosting the endogenous antioxidant system by upregulating the
expression of Nrf2 and its downstream targets may reduce elevated
oxidative stress and neuroinflammation [10,11].
The
elevated oxidative stress associated with AD may induce the
transcription of certain inflammatory factors such as nuclear factor
kappa B (NF-κB), which is a member of mitogen-activated protein kinase.
Studies have suggested that NF-κb plays an important role in the
accumulation of amyloid-beta and subsequent neurodegeneration [12,13].
Apart from oxidative stress, neuroinflammation is another feature of
neurodegenerative diseases executed by multiple factors, such as
activated astrocytes and microglial cells. Of note, it is reported that
oxidative stress is involved in the activation of astrocytes and
microglial cells [14,15].
The activation of microglia and astrocytes is one of core importance
for neuroinflammation and is involved in the pathogenesis of several
neurodegenerative diseases, such as AD and Parkinson’s disease (PD) [16,17].
Several brain regions are susceptible to AD-related pathology; the most
important one is the hippocampus, which is affected in the earlier
stage of AD. Hippocampus plays a role in the storage of long- and
short-term memories. Previous studies have extensively highlighted the
roles of growth factors and phosphatidylinositol 3-kinases
(PI3K)/protein kinase B (AKT) signaling pathways in hippocampal
plasticity and memory functions. The PI3K/AKT signaling pathway promotes
cell survival, proliferation, and differentiation, which are important
for normal cellular activities [18,19].
Brain-derived neurotrophic factor (BDNF) is considered the key factor
in the homeostasis of brain physiology and functions. This growth factor
support neuronal survival, synaptic functions, and induced hippocampal
neurogenesis and improve cognition [20,21].
A growing body of research shows that activations of neurotrophic
factors considerably reduce the effects of oxidative stress and
neuroinflammation [22,23].
Currently,
there is no known treatment available to cure AD, only certain drugs
may reduce the sign and symptoms associated with AD. Meanwhile, several
natural compounds such as flavonoids, vitamins, phenolic acids, and
polyphenols, have received special interest in the management of these
diseases. The natural compounds possess antioxidant and
anti-inflammatory activities and they also increase synaptic integrity,
memory, and cognitive functions [24,25].
Phenolic compounds, which possess antioxidant, anticancer,
antibacterial, and anti-inflammatory properties, are a group of
compounds mainly found in fruits and vegetables [26,27].
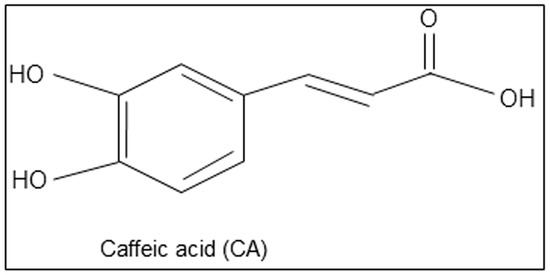
Here, we hypothesize that caffeic acid, a polyphenolic compound found
in vegetables, fruits, and herbs may reduce AD symptoms and its
pathological features. The chemical structure of caffeic acid is given
in Figure 1.


No comments:
Post a Comment