Ask your doctor where the EXACT PROTOCOL FOR USING THIS IS! If your doctor doesn't know, you don't have a functioning stroke doctor! Why the fuck are you seeing an incompetent doctor?
Anything done by your doctor with this earlier research? Why not?
hydrogels (15 posts to April 2012)
But is the peptide one better? Followup needed which will never occur.
Peptide-Based Scaffolds Support Human Cortical Progenitor Graft Integration to Reduce Atrophy and Promote Functional Repair in a Model of Stroke
Or nanofibers?
Nanofibrous scaffolds supporting optimal central nervous system regeneration: an evidence-based review
The latest here:
A Promising Application of Injectable Hydrogels in Nerve Repair and Regeneration for Ischemic Stroke
Authors Gao Y, Zhang TL, Zhang HJ, Gao J, Yang PF
Received 1 November 2023
Accepted for publication 13 December 2023
Published 12 January 2024 Volume 2024:19 Pages 327—345
DOI https://doi.org/10.2147/IJN.S442304
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Yuan Gao,1,2,* Ting-Lin Zhang,3,* Hong-Jian Zhang,1,2,* Jie Gao,3 Peng-Fei Yang1,2,4
1Oriental
Pan-Vascular Devices Innovation College, University of Shanghai for
Science and Technology, Shanghai, People’s Republic of China; 2School
of Health Science and Engineering, University of Shanghai for Science
and Technology, Shanghai, People’s Republic of China; 3Changhai Clinical Research Unit, Shanghai Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China; 4Neurovascular Center, Changhai Hospital, Naval Medical University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence:
Peng-Fei Yang, School of Health Science and Engineering, University of
Shanghai for Science and Technology, Shanghai, People’s Republic of
China, Tel +86-15921196312, Fax +86-2131161784, Email 15921196312@163.com
Jie Gao, Changhai Clinical Research Unit, Shanghai Changhai Hospital,
Naval Medical University, Shanghai, People’s Republic of China, Tel/Fax
+86-21-31162092, Email gaojiehighclea@smmu.edu.cn
Abstract:
Ischemic stroke, a condition that often leads to severe nerve damage,
induces complex pathological and physiological changes in nerve tissue.
The mature central nervous system (CNS) lacks intrinsic regenerative
capacity, resulting in a poor prognosis and long-term neurological
impairments. There is no available therapy that can fully restore CNS
functionality. However, the utilization of injectable hydrogels has
emerged as a promising strategy for nerve repair and regeneration.
Injectable hydrogels possess exceptional properties, such as
biocompatibility, tunable mechanical properties, and the ability to
provide a supportive environment for cell growth and tissue
regeneration. Recently, various hydrogel-based tissue engineering
approaches, including cell encapsulation, controlled release of
therapeutic factors, and incorporation of bioactive molecules, have
demonstrated great potential in the treatment of CNS injuries caused by
ischemic stroke. This article aims to provide a comprehensive review of
the application and development of injectable hydrogels for the
treatment of ischemic stroke-induced CNS injuries, shedding light on
their therapeutic prospects, challenges, recent advancements, and future
directions. Additionally, it will discuss the underlying mechanisms
involved in hydrogel-mediated nerve repair and regeneration, as well as
the need for further preclinical and clinical studies to validate their
efficacy and safety.
Keywords: ischemic stroke, central nervous system, hydrogels, neural restoration, nerve regeneration
Introduction
In the global context, ischemic stroke is one of the most prevalent and primary causes of disability and cognitive impairment.1 This condition is characterized by obstructions of blood vessels, leading to inadequate oxygenation and nutrition to the brain. This hypoxia/ischemia state triggers a series of neuropathological processes in tissues, causing substantial loss of brain parenchyma, diminished brain function, and profound neurological impairment.2 Ischemic stroke causes neuronal injury and death through three primary mechanisms. Firstly, it induces neurotoxic effects in glutamate, the primary neurotransmitter of the central nervous system (CNS), leading to severe neuronal death or damage.3 Secondly, neuronal damage is exacerbated by reactive oxygen species (ROS) generated due to mitochondrial dysfunction.4 Lastly, the inflammatory response triggered by ischemic stroke can also worsen nerve damage.5
As a result of ischemic stroke, neuron damage or death is particularly devastating, contributing to limited capacity for the CNS regeneration. It was historically believed that after a stroke, brain and CNS cells could not regenerate. However, evidence of neurogenesis in the adult human brain has emerged, demonstrating its occurrence in the hippocampal dentate nucleus and the subventricular region.6 Cells expressing markers associated with newborn neurons are found in the ischemic penumbra surrounding cerebral cortex infarction and tend to be predominantly situated near blood vessels.7
The addition of exogenous stem cells or therapeutic drugs to the affected region enhances their delivery and creates a favorable environment for the regeneration of damaged tissue.8 However, transplantation of stem cells and drugs can face challenges such as low survival rates and inadequate migration to the affected site. The mechanism through which stem cells contribute to nerve repair is more likely related to their ability to secrete various growth factors through paracrine action. These growth factors help to promote the endogenous brain nerve repair process, rather than solely relying on direct cell replacement. This paracrine signaling enables the stimulation of nearby cells to aid in the regeneration and recovery of damaged neural tissue. Delivering growth factors can indeed promote endogenous brain recovery,9 but one major obstacle is the limited diffusion of these molecules across the blood-brain barrier (BBB). To overcome this limitation, researchers have been exploring various strategies, such as using nanotechnology-based drug delivery systems, focused ultrasound to temporarily open the BBB, or even direct injection of growth factors into the brain. Hydrogels have garnered significant attention as an optimal framework for promoting cell proliferation and survival.
Hydrogels are described as a network of cross-linked polymeric units that form a 3D structure like the extracellular matrix (ECM) of native tissues. The ECM is a dynamic three-dimensional network of macromolecules that offer structural support for cells and tissues. Due to its inducible properties, the ECM is used as a source of injectable hydrogels for preparation in regenerative therapies.10 Hydrogels have the remarkable ability to hold a substantial amount of water within their structure, facilitating the transport of cells, drugs, and proteins, thus aiding in the repair of injured nerves. The injectable nature of hydrogels allows for precise targeting of therapeutic stem cells or small molecules to specific brain regions while minimizing invasiveness during the treatment process.11 The continuous expansion of tissue engineering, based on hydrogel, offers more possibilities for CNS regenerative therapy after ischemic stroke.
In the emerging field of hydrogel therapy for ischemic stroke, while some reviews have explored the advancements in the application of hydrogel biomaterials for the treatment of ischemic stroke, to the best of our knowledge, there is currently no comprehensive and systematic review that encompasses the various types of injectable hydrogels, design considerations for neural repair following stroke, and their underlying mechanisms of action. Therefore, there is a pressing need for a comprehensive summary and synthesis of the research progress concerning injectable hydrogels in the context of neural repair and regeneration for ischemic stroke, along with an examination of their potential applications, as well as the challenges they present. Such an effort will greatly facilitate our understanding of advanced therapies involving the design, engineering, and application of injectable hydrogels. This review introduces the classification and potential applications of hydrogels in nerve repair and regeneration after ischemic stroke, offering insights into the future possibilities for the clinical use and transformation of biomaterials (as shown in Figure 1).
Pathophysiological Mechanism of Nerve Injury in Ischemic Stroke
During cerebral artery embolization, blood flow to the corresponding brain region is impeded, leading to a significant reduction or blockage of blood supply. As a result, there is a lack of timely delivery of energy metabolites and oxygen to the affected area.12 After ischemic stroke, neurons experience disruptions in ATP synthesis, leading to energy deficiency. This deficiency causes an imbalance in ion gradients and results in the excessive release of excitatory amino acids, such as glutamic acid. Consequently, there is an influx of intracellular calcium (Ca2+), which triggers the activation of apoptosis and necrosis pathways, contributing to neuronal cell death.13 Ca2+ influx can stimulate the production of nitric oxide (NO), which, in turn, reacts with superoxide to form peroxynitrite. This peroxynitrite can lead to neurotoxicity, causing damage to neurons.14 Additionally, Ca2+ influx also contributes to an increase in the production of reactive oxygen species (ROS), primarily through oxidative phosphorylation in the mitochondria. The excessive ROS generation further exacerbates oxidative stress and cellular damage in the affected brain region.15 On one hand, ROS can have various effects on blood vessels and the blood-brain barrier, leading to nerve injury.16 ROS-induced oxidative stress can increase the permeability of the blood-brain barrier, making it more vulnerable to damage and potentially allowing harmful substances to enter the brain tissue.17 On the other hand, ROS can promote platelet aggregation, leading to the formation of blood clots, which can further impede blood flow and exacerbate ischemic damage. In addition, multiple signaling pathways participate in neuroinflammation in ischemic stroke.15 After ischemic stroke, the release of Damage-Associated Molecular Patterns (DAMPs) by injured or dead cells triggers neuroinflammatory responses. The neuroinflammatory response and the prolonged ischemia contribute to increased neuronal death and worsen the overall outcome after ischemic stroke.
After ischemia-reperfusion (I/R), blood flow recovery and reoxygenation may further aggravate brain tissue injury.18 After reperfusion, neutrophils, and other inflammatory cells in the blood infiltrate the ischemic area and release numerous inflammatory mediators, such as interleukin and nuclear factor-κB. These mediators facilitate the adhesion of inflammatory cells to endothelial cells, leading to the rupture and necrosis of endothelial cells. As a result, the blood-brain barrier is compromised, exacerbating the injury in the ischemia-reperfusion area.19 Moderate cerebral I/R injury can trigger autophagy and activate the cellular recovery system, allowing cells to survive and cope with the damage. In contrast, severe cerebral I/R injury primarily activates apoptosis and necrosis pathways, which can lead to significant cell death and exacerbate the overall injury.20
In regions surrounding an ischemic core area, cerebral blood flow (CBF) levels may be below functional thresholds required for normal cellular function. Despite this, they may still be temporarily above the threshold for cell death. This region is referred to as the penumbra area. The penumbra area is considered a critical zone where brain tissue has the potential to recover if blood flow is restored promptly and effectively. It is a target for therapeutic interventions aimed at salvaging brain tissue and reducing the extent of damage after ischemic stroke.21 Neuroprotective therapy is therefore primarily targeted at the penumbra, which represents potentially salvageable tissue.22 After I/R, the penumbra can be saved by regaining oxygen and nutrients, but it is undeniable that the nerve damage caused by I/R may also be difficult to recover.Traditional neuroprotection in stroke patients has focused primarily on mitigating the ischemic cascade reaction inside the affected brain area.23 In a new perspective, protection must focus on the entire neurovascular unit. The neurovascular unit comprises various cell types, including neurons, astrocytes, microglia, pericytes, and endothelial cells, all of which play crucial roles in maintaining the integrity and functionality of the brain’s blood vessels and neuronal network. By protecting the entire neurovascular unit, therapeutic strategies can address the complex interactions and interdependencies among these cell types, which collectively contribute to brain function and tissue repair. In the hours or days following a stroke, there is intercellular signaling between various components of the neurovascular unit, which can either amplify damage or promote protection of neurons. The interactions within the neurovascular unit are dynamic and can switch between these states (as shown in Figure 2).24 In the ischemic cascade, astrocytes and microglia respond to the “help me” signal from damaged neurons by releasing trophic factors, extracellular vesicles, and transferring mitochondria to support the neurons. Instead of solely targeting one specific component of the ischemic cascade, a promising approach is to employ cellular protection methods that can impact multiple aspects of the cascade. By considering the intricate interactions within the neurovascular unit, therapies can be designed to enhance the overall resilience of brain tissue to ischemic injury, leading to more comprehensive and effective neuroprotection.
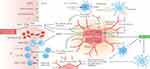 |
Figure 2 Ischemic cascade elements and mechanisms of neurovascular units in acute ischemic stroke. Notes: Printed with permission from Nature Publishing Group: Nature Reviews Neurology, Pharmacological brain cytoprotection in acute ischemic stroke — renewed hope in the reperfusion era, Marc Fisher, Sean I. Savitz. Copyright © Springer Nature Limited 2022.24 |
Hydrogels as carriers can effectively protect and maintain the bioactivity of stem cells and vesicles, creating a stable microenvironment at the injury site. This approach facilitates directed migration of stem cells and reconstruction of neurovascular units, thereby promoting effective nerve repair and regeneration. The integration of hydrogels with stem cells and vesicles offers a promising therapeutic strategy for targeted treatments of neurovascular units, leading to improved outcomes and rehabilitation for ischemic stroke patients.

No comments:
Post a Comment