This line in one of the 19 posts I have on cannabidiol should mean your doctors are jumping with joy to get this delivered to you. But you DON'T HAVE A FUNCTIONING STROKE DOCTOR , DO YOU?
CBD use has also improved learning and memory and increased blood flow in the cerebrum of individuals with cognitive decay.(This sounds very useful to us.)
Cannabidiol (19 posts to January 2016)
Nanosuspension-Loaded Dissolving Microneedle Patches for Enhanced Transdermal Delivery of a Highly Lipophilic Cannabidiol
Authors Cheng A, Zhang S, Meng F, Xing M, Liu H, Yang G, Gao Y
Received 1 December 2023
Accepted for publication 11 April 2024
Published 7 May 2024 Volume 2024:19 Pages 4061—4079
DOI https://doi.org/10.2147/IJN.S452207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Xing Zhang
Aguo Cheng,1,2 Suohui Zhang,1,3 Fanda Meng,4 Mengzhen Xing,5 Han Liu,3 Guozhong Yang,3 Yunhua Gao1– 3
1Key
Laboratory of Photochemical Conversion and Optoelectronic Materials,
Technical Institute of Physics and Chemistry of Chinese Academy of
Sciences, Beijing, People’s Republic of China; 2University of Chinese Academy of Sciences, Beijing, People’s Republic of China; 3Beijing CAS Microneedle Technology Ltd, Beijing, People’s Republic of China; 4School
of Clinical and Basic Medical Sciences, Shandong First Medical
University & Shandong Academy of Medical Sciences, Jinan, Shandong
Province, People’s Republic of China; 5Key Laboratory of New
Material Research Institute, Department of Pharmaceutical Research
Institute, Shandong University of Traditional Chinese Medicine, Jinan,
Shandong Province, People’s Republic of China
Correspondence:
Yunhua Gao, Key Laboratory of Photochemical Conversion and
Optoelectronic Materials, Technical Institute of Physics and Chemistry
of Chinese Academy of Sciences, Beijing, People’s Republic of China, Tel
+86 (10)82543581, Email yhgao@mail.ipc.ac.cn
Purpose:
Transdermal Drug Delivery System (TDDS) offers a promising alternative
for delivering poorly soluble drugs, challenged by the stratum corneum’s
barrier effect, which restricts the pool of drug candidates suitable
for TDDS. This study aims to establish a delivery platform specifically
for highly lipophilic drugs requiring high doses (log P > 5, dose
> 10 mg/kg/d), to improve their intradermal delivery and enhance
solubility.
Methods: Cannabidiol (CBD, log P = 5.91)
served as the model drug. A CBD nanosuspension (CBD-NS) was prepared
using a bottom-up method. The particle size, polydispersity index (PDI),
zeta potential, and concentration of the CBD-NS were characterized.
Subsequently, CBD-NS was incorporated into dissolving microneedles
(DMNs) through a one-step manufacturing process. The intradermal
dissolution abilities, physicochemical properties, mechanical strength,
insertion depth, and release behavior of the DMNs were evaluated.
Sprague-Dawley (SD) rats were utilized to assess the efficacy of the DMN
patch in treating knee synovitis and to analyze its skin permeation
kinetics and pharmacokinetic performance.
Results:
The CBD-NS, stabilized with Tween 80, exhibited a particle size of
166.83 ± 3.33 nm, a PDI of 0.21 ± 0.07, and a concentration of 46.11 ±
0.52 mg/mL. The DMN loaded with CBD-NS demonstrated favorable
intradermal dissolution and mechanical properties. It effectively
increased the delivery of CBD into the skin, extended the action’s
duration in vivo, and enhanced bioavailability. CBD-NS DMN exhibited
superior therapeutic efficacy and safety in a rat model of knee
synovitis, significantly inhibiting TNF-α and IL-1β compared with the
methotrexate subcutaneous injection method.
Conclusion:
NS technology effectively enhances the solubility of the poorly soluble
drug CBD, while DMN facilitates penetration, extends the duration of
action in vivo, and improves bioavailability. Furthermore, CBD has shown
promising therapeutic outcomes in treating knee synovitis. This
innovative drug delivery system is expected to offer a more efficient
solution for the administration of highly lipophilic drugs akin to CBD,
thereby facilitating high-dose administration.
Keywords: nanosuspension, highly lipophilic drugs, dissolving microneedle, cannabidiol, knee synovitis
Graphical Abstract:
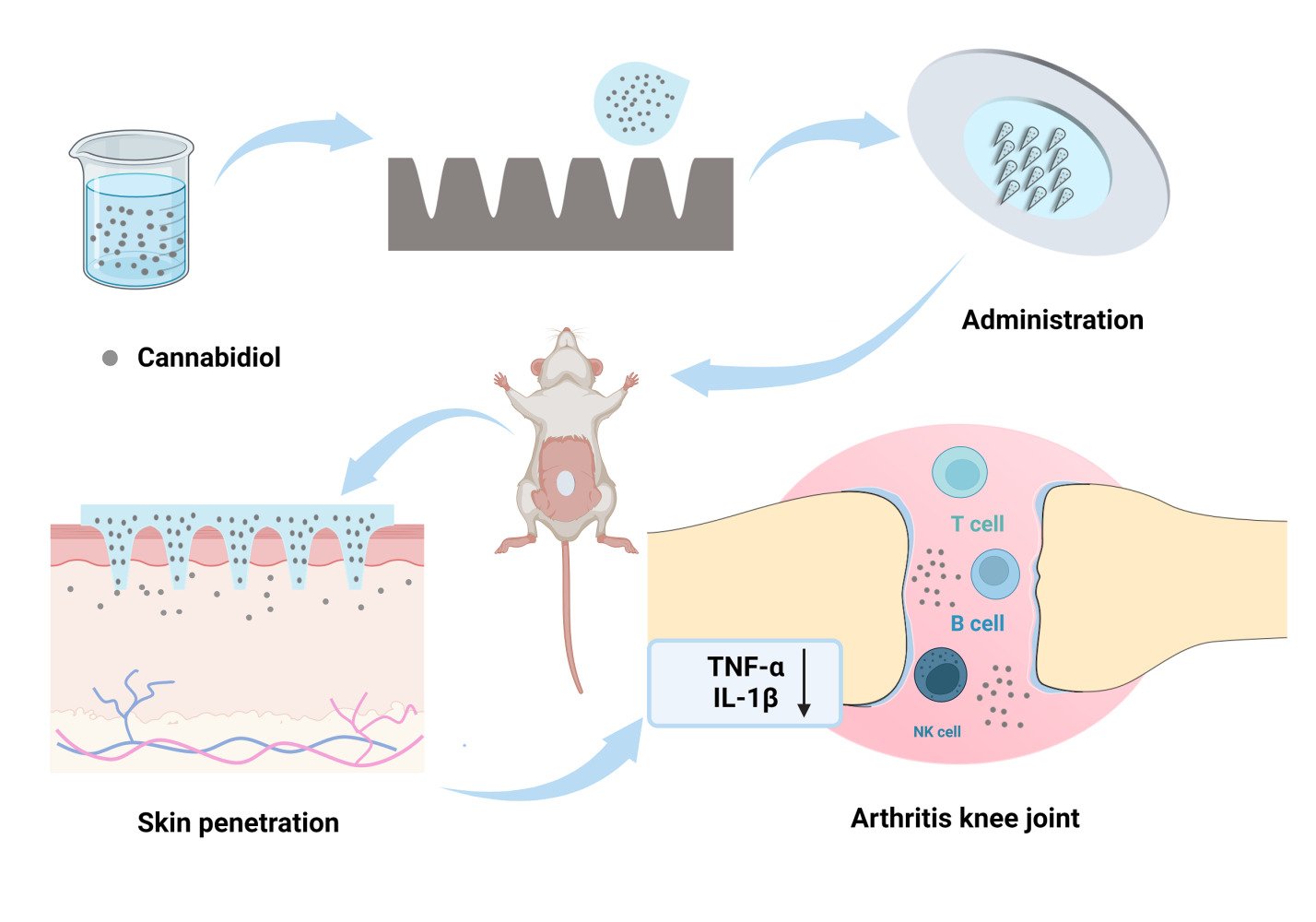
Introduction
The poor solubility of many current drugs and drug candidates presents a significant challenge within the pharmaceutical industry.1 Approximately 40% of recently marketed drug compounds exhibit limited solubility in water, complicating their development and production processes.2,3 The low solubility often leads to reduced dissolution rates, adversely affecting the bioavailability and absorption of orally administered drugs.4,5
To exploit the therapeutic potential of poorly soluble drugs, several solubility enhancement strategies are employed, including nanoparticle systems, solubilization with cosolvents and surfactants, amorphous forms, solid dispersions, cocrystals, polymeric micelles, inclusion complexes, pH adjustments, salt forms, and liposomes.6,7 Beyond mere solubilization, research is increasingly directed toward advanced delivery systems aimed at not only improving the bioavailability of poorly soluble agents but also enhancing patient compliance.8 These systems address issues such as frequent dosing, swallowing difficulties, and needle phobia, offering controlled, targeted, or regulated delivery. Among these, the transdermal drug delivery system (TDDS) has garnered significant interest.9
TDDS enable drugs to bypass the liver’s first-pass effect by penetrating the skin barrier into systemic circulation, often requiring lower therapeutic doses than traditional methods.10 They allow for controlled drug release, maintaining stable plasma concentrations, which can mitigate side effects and variability among patients. This route simplifies administration without medical personnel and reduces the influence of gastrointestinal pH variations.11,12 However, the barrier effect of the stratum corneum (SC) and the limited number of hair follicles and sebaceous glands restrict TDDS effectiveness, especially for drugs with high lipophilicity.13 Traditionally, ideal candidates for TDDS are those with a molecular weight of less than 500 Da, a log P value between 1 and 3, and a daily dose of less than 10 mg/kg.14,15 This narrows the scope of drugs suitable for TDDS, particularly for highly lipophilic drugs (log P > 5), whose transdermal delivery is hampered by low permeability.16 To overcome these limitations, various passive and active technologies have been developed to enhance TDDS penetration and expand the range of suitable drug candidates.10
Microneedles (MNs) are emerging as scalable and commercially viable technology.17 As third-generation TDDS, they typically ranging from 150 to 1500 μm in length and can penetrate the SC of the skin to create micron-sized pores for drug delivery.18,19 For poorly soluble drugs, dissolving microneedles (DMNs) are particularly advantageous for long-term treatment due to their excellent biodegradability and compatibility.20,21 However, the integration of poorly soluble drugs into the aqueous solution of DMN matrix materials often faces challenges, such as uneven drug dispersion within the DMN matrix.22 Additionally, the loading dose of drugs in DMNs is often frequently constrained by their low solubility. The use of organic solvents to enhance drug solubility may also raise safety concerns regarding MNs.23 Consequently, for highly lipophilic drugs that require high doses, developing a method for achieving homogeneous dispersion in MNs, thereby ensuring sustained delivery and improved bioavailability, is crucial.
Nanosuspensions (NS), or nanocrystals (NC), are proposed as a promising solution to this challenge. They facilitate a more uniform particle size distribution for lipophilic drugs when combined with DMN.24,25. Compared to other systems like nanoliposomes, nano micelles, and self-emulsifying systems, NS offers a high drug-loading capacity with minimal use of surfactants.26–28 NS can create a concentration gradient with the epidermal layer, enhancing passive diffusion; it also has potential for hair follicle targeting, making it conducive to forming a reservoir for continuous drug delivery.29
More at link.
Cannabidiol (CBD, log P = 5.91) served as a model drug to demonstrate the combined use of DMN and NS technology for high-dose, sustained intradermal delivery of highly lipophilic drugs (Figure 1). CBD is a non-psychoactive and essentially non-toxic compound derived from the cannabis plant.30 It has been approved by the FDA for treating refractory epilepsy and exhibits promising therapeutic effects in analgesia, inflammation, and neurodegenerative disorders.31–33 The interaction of CBD with CB2 receptors, primarily found in immune cells, leads to reduced inflammatory responses, positioning it as a potential treatment for synovitis-associated pain and inflammation.34 Additionally, CBD has been shown to suppress the release of pro-inflammatory cytokines, such as TNF-α and IL-6. In various conditions like collagen-induced arthritis, osteoarthritis, monoarthritis, and carrageenan-induced joint swelling, CBD has shown effective therapeutic outcomes with minimal side effects.35–38 Despite its general tolerance and minimal side effects, CBD’s hydrophobic nature and unstable absorption in the gastrointestinal system can lead to unpredictable pharmacokinetics.10,39,40 Oral administration of CBD is particularly affected by significant first-pass metabolism in the liver, resulting in approximately only 6% bioavailability.41 These limitations substantially restrict CBD’s therapeutic application in clinical settings.
 |
Figure 1 Chemical structures of CBD. |
In this study, the bottom-up method was used to reduce the particle size of CBD, enhancing solubility through CBD-NS, which was then incorporated into DMN. The dissolution, physicochemical, and mechanical properties of DMN were characterized, and the release behavior in vitro of CBD-NS DMN was evaluated. Sprague-Dawley (SD) rats were used to assess the efficacy of the DMN patch in treating knee synovitis, along with its skin permeation kinetics and pharmacokinetic profiles. The aim of this work was to provide a platform for highly lipophilic drugs that require high-dose administration and contribute to improving their skin permeability and bioavailability.
No comments:
Post a Comment