Why won't your doctor use this to figure out how to get you 100% recovered?
Laziness? Incompetence? Or just don't care? No leadership? No strategy? Not my job?
Neuron matters: neuromodulation with electromagnetic stimulation must consider neurons as dynamic identities
Journal of NeuroEngineering and Rehabilitation volume 19, Article number: 116 (2022)
Abstract
Neuromodulation with electromagnetic stimulation is widely used for the control of abnormal neural activity, and has been proven to be a valuable alternative to pharmacological tools for the treatment of many neurological diseases. Tremendous efforts have been focused on the design of the stimulation apparatus (i.e., electrodes and magnetic coils) that delivers the electric current to the neural tissue, and the optimization of the stimulation parameters. Less attention has been given to the complicated, dynamic properties of the neurons, and their context-dependent impact on the stimulation effects. This review focuses on the neuronal factors that influence the outcomes of electromagnetic stimulation in neuromodulation. Evidence from multiple levels (tissue, cellular, and single ion channel) are reviewed. Properties of the neural elements and their dynamic changes play a significant role in the outcome of electromagnetic stimulation. This angle of understanding yields a comprehensive perspective of neural activity during electrical neuromodulation, and provides insights in the design and development of novel stimulation technology.
Introduction
As electrically excitable cells, neurons transfer information among each other using synapses or electric connections. Fluctuations of the transmembrane potential and dynamic changes in the voltage-dependent ion channels produce action potentials and induce synaptic connections with other neurons. These unique features of the neurons, therefore, allow the exogenous electrical signals to control or modify their excitability, behavior, and functions. Numerous electrical stimulation devices have been invented to deliver electric currents that target the brain and spinal cord for neuromodulation. Among these many clinical successes, deep brain stimulation (DBS) uses implanted electrode leads. DBS is an established symptomatic surgical therapy for Parkinson’s disease, essential tremor, and several other movement and neuropsychiatric disorders [1]. Transcranial magnetic stimulation (TMS), an electromagnetic technology, induces electric currents to control neural activities inside the brain [2]. Transcranial direct-current stimulation (tDCS) uses weak, direct currents to shift the resting potential of cortical neurons in a non-invasive manner. Spinal cord stimulation (SCS) uses minimally invasive stimulation for the treatment of chronic neuropathic pain [3]. From the start of these clinical practices with electrical neuromodulation, researchers began to ask the questions, “What determines the outcome of electrical stimulation? How can we improve it?”.
From the early years of its practice, researchers have realized that optimal control of neural activity with the electric field seemed largely dependent on the properties of the electric currents that were applied to the target neuronal tissue. Many key parameters that define the electric current have been exclusively studied, such as intensity, duration, frequency, and orientation [4,5,6]. For example, neurons in the motor cortex displayed different sensitivities to transcranial magnetic fields with differing coil orientations, shapes of the induced current pulse, and intensities [7,8,9,10,11,12]. Development of various technologies has allowed researchers to link various stimulation parameters to fundamental cellular physiology, such as the excitation of individual neurons [13], changes in ion channel dynamics [14], and alternation of synaptic transmission [15, 16], etc. Cellular analysis reveals that the orientation of the magnetic field determines the geometric pattern of membrane polarization on the neuron [13, 17]. To achieve optimal outcomes, clinical research witnesses the continuous optimization of the stimulating parameters [18,19,20]. In TMS, with careful coil design and placement around the head, the magnetic field can eventually achieve improved clinical outcomes [10, 11].
Even though substantial variance has been observed in patients and normal subjects, at the individual neuron level, the impacts of the targeted tissue and neurons on the outcome of the electrical stimulation has drawn far less attention. For example, repetitive transcranial magnetic stimulation (rTMS) over the occipital cortex led to a significantly increased visual cortex excitability in subjects affected by migraine with aura, but a decreased visual cortex excitability in normal subjects [21]. At the cellular level, the same electrical stimulation protocol can cause significant difference in neural activity. Identical stimulation parameters can result in neuronal activation, suppression, or both, depending on the brain region [22]. Considering the complicated interactions between the applied electric field and the biological tissue (reviewed in [23]), it is essential to understand the outcome of electrical stimulation and its dependence on both the properties of the stimuli and its neural targets.
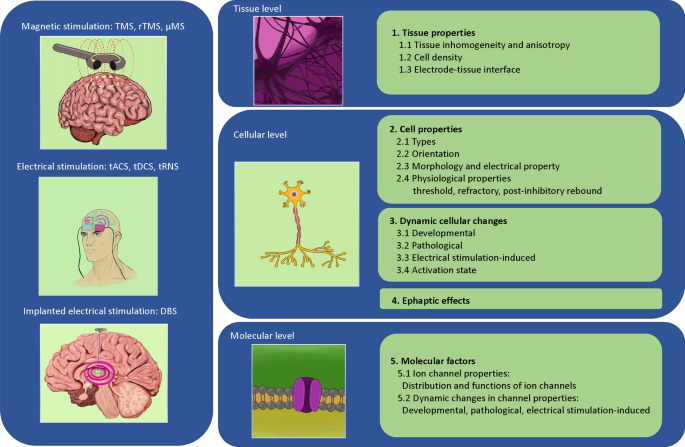
This paper will review the impact of biological complexity on the outcomes of neuromodulation with electromagnetic stimulation. We organize our evidence in a multidimensional format—at the tissue level, cell level, and molecular/ion channel level, respectively (Fig. 1). It is our hope that reviewing these biological complexities during electrical stimulation will provoke awareness in the scientific community during the investigation of the underlying mechanisms of stimulation, and will facilitate the design and development of novel protocols and technologies for neural control with electrical stimulation.
Conceptual figure that summarizes multiple different electromagnetic stimulation modalities across different biological levels. The text of the paper is organized to provide evidence for how the different factors at each of these levels could impact the outcome of the stimulation. Abbreviations are provided separately in the “abbreviations list” of the text
Review
Dynamic changes in tissue properties impact neural stimulation with electromagnetic fields
Biophysical complexity of the neural tissue that surrounds the stimulating electrode alters the electric current distribution and the stimulation outcome. This includes, but is not limited to, inhomogeneity and anisotropy of the tissue, compactness of the cell, and impedance change due to electrode-tissue interaction.
Impact of tissue inhomogeneity and anisotropy on electrical stimulation
Under electrical stimulation, distribution of the electric current inside the neural tissue is largely dependent on the electrical properties of the tissue, including homogeneity and isotropy. A homogeneous material has the same properties at every point. An isotropic material has the same physical properties in all directions. Most neural tissues are inhomogeneous, compositing different types of cells, tissues, fat, irregular vascularization, pH variation, and heterogeneous oxygen concentration, etc. Inhomogeneous resistance is present in the extracellular space in many brain regions, such as the hippocampus and the cerebellum [24]. For example, the tissue conductivity can vary in different layers of the hippocampus [25], with the resistance in the stratum pyramidale two times higher than other hippocampal layers. Additionally, tissue swelling has been observed after intense neural activity, which could lead to an increase in extracellular resistance [26]. Similarly, directional variance in electrophysical properties (anisotropy) is frequently observed in biological tissue. This material property allows tissues to change or assume different properties in different directions. For example, the specific direction of a fiber track generates anisotropy in the brain [27].
Under electrical stimulation, the electric field could be significantly dispersed by non-homogenous tissue conductivity and anisotropy. A recent numerical study [28] suggested that a decrease in tissue conductivity resulted in a smaller volume of neuronal tissue being activated by an electrode. Anisotropic properties of the head play significant roles in the distribution of the electric fields and the effects of transcranial direct-current stimulation (tDCS) [29, 30], since the electric current tends to flow in directions more parallel to the white matter fiber tracts.
In magnetic stimulation, electric currents inside the tissue are generated by electromagnetic induction, which can also be significantly dispersed by the inhomogeneity and anisotropy of the tissue. During TMS, the electric field and current density distribution induced in the brain were significantly altered by the tissue [27, 31, 32]. Specifically, it was found that tissue types and fiber orientation affect the induced electric field [32] and the outcome of the treatment [27].
Impact of cells and cell density on electrical stimulation
The cell membrane is largely non-conductive. Consequently, the presence of the cells will inevitability perturb the externally-applied electric field.
At the single cell level, a spherical cell disturbs the electric potential generated by a microelectrode [33], or a magnetic field [13]. This altered distribution of the microscopic electric field around a single cell could cause secondary effects to the neighboring cells. For example, it will change the membrane potentials in the neighboring cell without direct physical contact between the two cells [34]. Normally, a single cell is surrounded by many other cells. Therefore, although it is possible to predict the polarization and excitability of a single neuron under a specific stimulation electrode arrangement [33], it is nearly impossible to describe the polarization patterns of a single neuron embedded in a large cluster of cells. Indeed, polarization in individual cells depends on the overall density and spatial arrangement of the cells in the three-dimensional cell cluster [35].
Impact of impedance change on electrical stimulation at the electrode-tissue interface
When electric currents are delivered by the implanted electrode for neural stimulation, the impedance between the electrode and the contacting tissue determines the electric current flow inside the tissue [36]. However, impedance changes during chronic implantation. The devastated path left by an intracortical-inserted electrode can lead to vascular damage and tearing of the extracellular matrix, damaging both neurons and glial cells [37, 38]. Consequently, increased electrode-tissue impedance is observed in the weeks following electrode implantation in the brain, stabilizing at approximately 3 to 6 months. Postmortem studies from patients confirmed the presence of a fibrous sheath, astrocytosis, neuronal loss, and neuroinflammation in the immediate vicinity of the electrode [39]. Effects of glial scar formation around the electrode also prevent the readout of nerve activity [40]. In a study that investigated the relationship between impedance change and neural recording in a set of chronically implanted animals, the authors found a positive correlation between glial cells and tissue impedance, and a negative correlation between glial cell density and neural signal quality [41]. Establishing how electrical stimulation influences the electrical and histological properties of the surrounding tissue is essential for the development of future neuromodulation systems for more efficient stimulation.
Cell properties impact neural stimulation with electromagnetic fields
Different cells respond differently to the same electrical stimulation, due to the variabilities in the morphology, biophysical properties, and physiology among different neuronal populations.
More at link.
No comments:
Post a Comment