But why go thru all the trouble of stem cells if exosomes are the reason for the benefits? Which must be why no one seems to be monitoring stem cell survival.
Application of stem cell-derived exosomes in ischemic diseases: opportunity and limitations
The latest here:
Meso-Brain project explores 3D printed stem cells to treat neurological conditions
A stem cell research project headed up by Aston University is developing 3D nanoprinting techniques that they claim could revolutionize neuroscience and the treatment of diseases such as Parkinson’s and dementia.
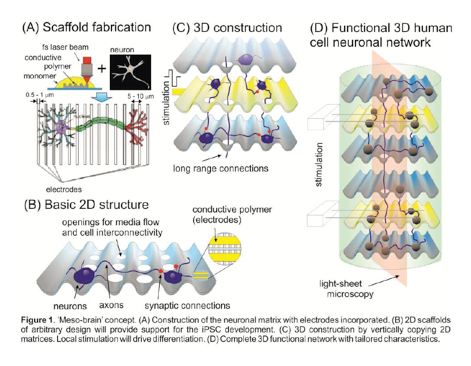
The EU-funded Meso-Brain project aims to generate customizable 3D printed networks of stem-cell-derived neurons to produce a new generation of accurate modeling and testing tools.
The project hopes to address the limitations of current neuronal culturing techniques by combining cutting-edge research within human stem cell biology, nanoscale 3D printing, computational network modeling and light sheet microscopy to discover novel treatment options for the long-term alleviation of brain dysfunction.
Meso-Brain hopes to unlock meaningful and practicable insights into the functioning of the brain, and will eventually allow researchers to accurately model brain networks more realistically than ever before.
Cell 3D printing
Stem cells generally serve as a repair system for the body, and, being unspecialized, are able to develop into a variety of different types of cells. As such, stem cells can develop into specialized cells such as blood, muscle, and brain cells, when required.
Cell 3D printing is an area that is receiving growing interest from researchers and 3D printing firms alike as a means of harnessing these desirable properties, particularly for regenerative medicine and bioprinting applications.
For instance, 3D printer OEM 3D systems announced a breakthrough in its Print to Perfusion bioprinting platform earlier this year, which can now rapidly produce full-size human lung scaffolds that can be perfused with living cells to create tissues. Meanwhile, scientists at the University of Buffalo have developed a new 3D bioprinting method that reduces the time needed to create cell-laden hydrogel structures, potentially bringing 3D printed organs closer to reality.
Elsewhere, researchers from the University of New South Wales have developed a novel technique to 3D print bone-mimicking structures containing living cells with potential uses for bone tissue engineering and disease modeling, and a new bioink created by Lund University is capable of supporting new cell and blood vessel growth once transplanted into new material.
In a similar vein to the Meso-Brain project, 3D bioprinting has previously been deployed by medical tech company Fluicell, R&D firm Cellectricon, and the Karolinska Institutet university to arrange neural brain cells into complex patterns in order to model the progress of neurological diseases.
The Meso-Brain project
The project was first launched by Aston University in 2016 with the goal of using nanoscale 3D printing to replicate the brain’s neural networks. The project has since received further funding from the EU’s Horizon 2020 FET-Open program to accelerate the pace of neuroscience research and pharmaceutical drug discovery.
Coordinated by Aston University, the project is made up of six partners from three countries, including human cell culture specialist Axol Bioscience, digital service provider Kite Innovation, the Institute of Photonic Sciences (ICFO), the University of Barcelona, and LZH Laser Zentrum Hannover E.V.
Meso-Brain combines “revolutionary” tools for micro-fabrication, neuronal network development and monitoring, and functional analysis to bring to light 3D human neuronal networks with tailored characteristics.
Through Meso-Brain, the consortium is working on developing a new type of neural culture and interacting interface system integrated with conductive polymers, that will facilitate electrical stimulation and recording of individual cells.
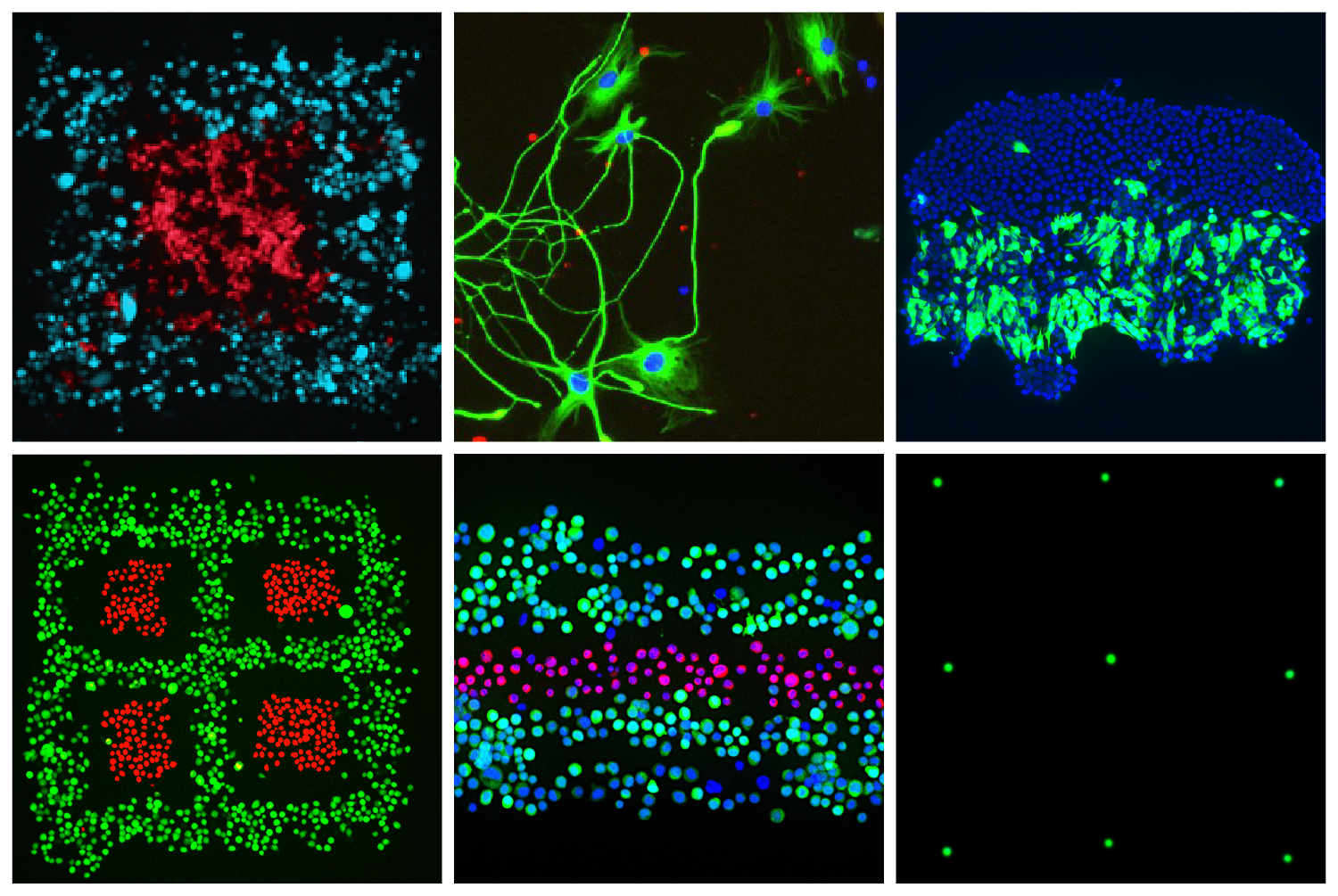
In naturally developing circuits in the brain, neurons and connections are first generally configured and then gradually refined over time in response to chemical and electrical activity. To replicate this process in the researchers’ own 3D printed scaffolds, neurons and astrocytes derived from stem cells are developed at specific cytophilic points through the use of chemical messages and electrical activity to promote and drive functional network development.
After this process, functional connectivity maps are drawn using newly-developed mathematical formulations to verify the function of the 3D printed neural network structure.
The customizable properties of the 3D printed scaffolds enable fluorescence imaging and interrogation with photonic and optical approaches, therefore making it possible to see how the neurons interact with each other in real-time.
Future impacts of Meso-Brain
It is hoped that the research developments within Meso-Brain will allow researchers to accurately and dynamically model brain networks to identify neurons in various states of dysfunction and test their reaction to new medicines and other treatments.
According to the project partners, the development of human 3D neuronal networks that exhibit physiologically relevant and reproducible architecture and activity could be “foundational” to the scientific community, enabling large-scale scientific investigation of human brain network function.
The project’s results also hope to facilitate large-scale pharmaceutical testing on human cells and human disease models with stem cells derived from patients, eventually leading to advances in neural transplantations for central nervous system therapy and repair.
Ultimately, the researchers believe Meso-Brain can aid the understanding and treatment of a range of neurological conditions such as dementia, Parkinson’s, and brain trauma.
No comments:
Post a Comment