You can see that semaphorins have been researched for years in stroke but obviously since we have NO LEADERSHIP AND NO STRATEGY nothing useful for stroke recovery has occurred with all this research. Leaders would solve stroke, not let it fester for decades.
Semaphorins and their Signaling Mechanisms January 2018
From there:
Early studies revealed that semaphorins function as axon guidance molecules,(We need this to have our white matter do the connections needed.) but it is now understood that semaphorins are key regulators of morphology and motility in many different cell types including those that make up the nervous, cardiovascular, immune, endocrine, hepatic, renal, reproductive, respiratory and musculoskeletal systems, as well as in cancer cells.
Astrocyte-Derived Exosomes Treated With a Semaphorin 3A Inhibitor Enhance Stroke Recovery via Prostaglandin D2 Synthase September 2018
Following experimental stroke, the recovering brain is vulnerable to lipoxygenase‐dependent semaphorin signaling October 2012
Serum semaphorin 7A is associated with the risk of acute atherothrombotic stroke February 2019
Use of semaphorin-4D binding molecules to promote neurogenesis following stroke May 2012
Ischemic neurons prevent vascular regeneration of neural tissue by secreting semaphorin 3A June 2011
Sustained
up-regulation of semaphorin 3A, Neuropilin1, and doublecortin
expression in ischemic mouse brain during long-term recovery February 2008
Cellular and molecular mechanisms of neural repair after stroke: making waves April 2006
The latest here:
Semaphorins in Adult Nervous System Plasticity and Disease
- 1Laboratory for Neuroregeneration, Netherlands Institute for Neuroscience, Royal Academy of Arts and Sciences, Amsterdam, Netherlands
- 2Department of Neuroscience Rita Levi-Montalcini and Neuroscience Institute Cavalieri Ottolenghi, University of Turin, Turin, Italy
Semaphorins, originally discovered as guidance cues for developing axons, are involved in many processes that shape the nervous system during development, from neuronal proliferation and migration to neuritogenesis and synapse formation. Interestingly, the expression of many Semaphorins persists after development. For instance, Semaphorin 3A is a component of perineuronal nets, the extracellular matrix structures enwrapping certain types of neurons in the adult CNS, which contribute to the closure of the critical period for plasticity. Semaphorin 3G and 4C play a crucial role in the control of adult hippocampal connectivity and memory processes, and Semaphorin 5A and 7A regulate adult neurogenesis. This evidence points to a role of Semaphorins in the regulation of adult neuronal plasticity. In this review, we address the distribution of Semaphorins in the adult nervous system and we discuss their function in physiological and pathological processes.
Introduction
The development of complex tissues depends on proliferation, differentiation and migration of cells. Cell guidance cues regulate these events and continue to be essential throughout life to maintain tissue homeostasis. Semaphorins constitute a large family of cell guidance cues, which are present in some viruses and conserved across animal species, from worms and flies to humans. Thirty Semaphorin proteins have been identified so far. They can be divided into eight classes (Sema1-7 and the viral Semaphorins, SemaV) on the basis of phylogenetic relationships and structural features. Sema1, Sema2, and Sema5C are found in invertebrates, whereas all the other Semaphorin classes are found in vertebrates (Bamberg et al., 1999; Pasterkamp, 2012; Alto and Terman, 2017; Figure 1). Semaphorins can be secreted (Sema2, Sema3, and SemaV), membrane-spanning (Sema1, Sema4, Sema5, and Sema6) or glycosylphosphatidylinositol-anchored (Sema7A). The structural hallmark of the Semaphorin protein family is an extracellular domain at the N-terminal region, important for dimerization and interaction specificity, called Sema domain, which is followed by a Plxn–Semaphorin–integrin domain and by distinct protein domains that further define Semaphorins (Zhou et al., 2008; Figure 1).
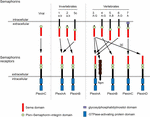
Figure 1. Semaphorins and their receptors. Semaphorins can be categorized into eight classes. Viral Sema is found in the genomes of certain DNA viruses; Sema1, Sema2, and Sema5c comprise the invertebrate Semaphorins; the other Semaphorin classes are found in vertebrates. Semaphorins are secreted (viral Sema, Sema2, and Sema3), membrane-spanning (Sema1, Sema4, Sema5, and Sema6) or glycosylphosphatidylinositol-anchored proteins (Sema7A). Semaphorins bind to Plxn receptors (PlxnA1–PlxnA4, PlxnB1–PlxnB3, PlxnC1, and PlxnD1) – see arrows for specific interactions. Sema3 require Npn for binding to PlxnA.
First characterized by their ability to act as repulsive cues for growing neurites (Kolodkin et al., 1992, 1993; Luo et al., 1993), Semaphorins are now known to be crucial molecules also for the development and functioning of the musculoskeletal, cardiovascular, respiratory, immune, endocrine, reproductive, hepatic, and renal system. In addition, Semaphorin signaling has been linked to diseases affecting these systems, as well as to cancer (Roth et al., 2009; Neufeld et al., 2012; Pasterkamp, 2012; Tamagnone, 2012; Giacobini and Prevot, 2013; Kang and Kumanogoh, 2013; Kumanogoh and Kikutani, 2013).
The effects of Semaphorins occur through binding to their receptors, the neuropilin (Npn) and plexin (Plxn) protein families (Figure 1). Plxns are grouped in four classes, from A to D, with four A-type, three B-type, one C-type and one D-type. The Plxn extracellular region contains several sema domains, which are important for binding to Semaphorins, whereas the intracellular region contains GTPase-activating protein domains (Takahashi et al., 1999; Tamagnone et al., 1999). In general, Semaphorins exist as homodimers, both in an unbound state and when interacting with Plxns. Semaphorin homodimers bring together two Plxn monomers or disrupt existing Plxn homodimers, relieving Plxn autoinhibition, which might be caused by an interaction between the sema domain of Plxn and the rest of the Plxn extracellular domain (Takahashi and Strittmatter, 2001; Kong et al., 2016). Once activated, Plxn signals through downstream molecules, including GTPases of the Rho family, protein kinases such as MAPK, and enzymes such as MICAL (molecule interacting with casL), which induce the phosphorylation of intracellular proteins of the collapsin responsive mediator protein (CRMP) family (Vikis et al., 2000; Hu et al., 2001; Liu and Strittmatter, 2001; Terman et al., 2002; Pasterkamp et al., 2003; Hota and Buck, 2012). CRMPs, in turn, affect actin and microtubule dynamics (Hung et al., 2010, 2011; Alto and Terman, 2017). Membrane-associated Semaphorins can also act as receptors or co-receptors for Semaphorins located on other cells, a phenomenon known as reverse signaling (Battistini and Tamagnone, 2016).
Class 3 Semaphorins require Npn as co-receptors (Npn-1 and -2). Npn-1 homodimers function as ligand-binding receptors for Sema3A and Sema3D; Npn-2 homodimers as receptors for Sema3F; and Npn-1 and Npn-2 heterodimers as receptors for Sema3B, 3C, 3E, and 3G (He et al., 2019; Toledano et al., 2019). Npn are transmembrane proteins with short intracellular domains that lack intrinsic enzymatic or signaling activity. They do not seem to act as a direct bridge between Plxn and Semaphorins but may function in the presentation of Semaphorin to Plxn. In addition, Npn can bind vascular endothelial growth factor (VEGF) in co-receptor complexes with VEGF receptors (Kruger et al., 2005; Pasterkamp, 2012), regulating blood and lymphatic vessel growth (Tammela et al., 2005).
Additional receptors can directly bind Semaphorins, including CD72 (Kumanogoh et al., 2000), Tim2 (Kumanogoh et al., 2002), and integrins (Pasterkamp et al., 2003). Moreover, co-receptors that associate with Sema binding receptors affect the signaling outcome of Sema-receptor interactions (Sharma et al., 2012). Cell adhesion molecules, such as Nr-CAM and L1 CAM can associate with Npn receptors and can be required for transducing class 3 Sema signals (Castellani et al., 2000, 2004; Falk et al., 2005). In addition, a number of receptor tyrosine kinases, such as VEGF receptor 2, Met, ErbB2 and off-track, associate with Plxns and Npns and become transactivated upon Sema binding (Sharma et al., 2012). Interestingly, Semaphorin function can be modulated by binding to proteoglycans (Kantor et al., 2004; de Wit et al., 2005; Zimmer et al., 2010; Cho et al., 2012; Dick et al., 2013). For example, class 5 Semaphorins demonstrate axon repulsive properties on neurites that co-express chondroitin sulfate proteoglycans and Plxns, while they turn into attractive cues if neurites express heparan sulfate proteoglycans adjacent to Plxns (Kantor et al., 2004).
Semaphorins have been discovered in the early 1990s as repulsive axon guidance molecules, enabling axons to find their targets and thus contributing to nervous system development (Kolodkin et al., 1992, 1993; Luo et al., 1993). In the peripheral nervous system, Semaphorins of several classes form molecular boundaries to prevent axons of dorsal root ganglion neurons, cranial nerves, spinal motoneurons or sympathetic neurons from entering inappropriate areas (Masuda and Taniguchi, 2016). Repulsive Semaphorin signaling is also crucial in the control of axon pathfinding of several classes of central nervous system (CNS) neurons during development (Sahay et al., 2003; Kolk et al., 2009; Pignata et al., 2016; Alto and Terman, 2017; Okada et al., 2019). The main mechanism how Semaphorins act as guidance molecules is through activation of Plxn signaling, which induces cytoskeletal changes in the growth cone of developing axons, such as depolymerization of actin filaments, attenuation of microtubule dynamics, and collapse of microtubule arrays (Goshima et al., 1997; Fritsche et al., 1999; Hung et al., 2010).
In the last three decades, Semaphorins have been shown to be involved in many other developmental processes that shape the nervous system, including axon defasciculation (Kolodkin et al., 1992; Tran et al., 2007; Claudepierre et al., 2008; Pecho-Vrieseling et al., 2009; Imai, 2012; Assens et al., 2016), lamina-specific patterning of synaptic connectivity (Skutella and Nitsch, 2001; Pasterkamp, 2012; Xie et al., 2019), axon terminal branching (Bagnard et al., 1998; Bagri et al., 2003; Dent et al., 2004; Cioni et al., 2013; Jung et al., 2019), dendritic morphogenesis and arborization (Polleux et al., 2000; Fenstermaker et al., 2004; Vodrazka et al., 2009; Ng et al., 2013; Cheadle and Biederer, 2014; Yamashita et al., 2014; Danelon et al., 2020), synapse formation (Godenschwege et al., 2002; Morita et al., 2006; Paradis et al., 2007; Yamashita et al., 2007; Tran et al., 2009; Kuzirian et al., 2013; Inoue et al., 2018; McDermott et al., 2018), subcellular target recognition by specific axons (Telley et al., 2016), pruning (Bagri et al., 2003; Sahay et al., 2003; Faulkner et al., 2006; Low et al., 2008; Uesaka et al., 2014), and removal of ectopic synapses (O’Connor et al., 2009; Tran et al., 2009; Mohan et al., 2018, 2021).
Many excellent reviews have addressed the functions of Semaphorins during nervous system development. Here, we will provide an overview of the role of Semaphorins in adult CNS physiology and pathology, including the role of Sema3A in plasticity processes through its interaction with the extracellular matrix (ECM).
Semaphorins in Adult Nervous System Physiology
Semaphorins are found in the nervous system not only during development but also in adulthood. Early studies showed that Sema3A mRNA expression persists in several discrete areas of the adult CNS and PNS (Luo et al., 1993; Giger et al., 1996, 1998; Pasterkamp et al., 1998; de Wit and Verhaagen, 2003). Since then, the role of Sema3A and other Semaphorins in the physiology of the adult nervous system has been progressively unveiled, pointing to a role of these axon guidance cues in the regulation of neuroplasticity.
 Daniela Carulli
Daniela Carulli Fred de Winter
Fred de Winter Joost Verhaagen
Joost Verhaagen
No comments:
Post a Comment