I couldn't tell a damn thing from the result or conclusion descriptions. Were the subjects able to recover functionality? If you can't answer that question, what the fuck was the point of the research?
Precise quantification of the time course of voluntary activation capacity following Botulinum toxin injections in the biceps brachii muscles of chronic stroke survivors
Abstract
Background
Spasticity is a key motor impairment that affects many hemispheric stroke survivors. Intramuscular botulinum toxin (BT) injections are used widely to clinically manage spasticity-related symptoms in stroke survivors by chemically denervating muscle fibers from their associated motor neurons. In this study, we sought to understand how BT affects muscle activation, motor unit composition and voluntary force generating capacity over a time period of 3 months. Our purpose was to characterize the time course of functional changes in voluntary muscle activity in stroke survivors who are undergoing BT therapy as part of their physician-prescribed clinical plan.Method
Our assessment of the effects of BT was based on the quantification of surface electromyogram (sEMG) recordings in the biceps brachii (BB), an upper arm muscle and of voluntary contraction force. We report here on voluntary force and sEMG responses during isometric elbow contractions across consecutive recording sessions, spread over 12 weeks in three segments, starting with a preliminary session performed just prior to the BT injection. At predetermined time points, we conducted additional clinical assessments and we also recorded from the contralateral limbs of our stroke cohort. Eight subjects were studied for approximately 86 experimental recording sessions on both stroke-affected and contralateral sides.Results
We recorded an initial reduction in force and sEMG in all subjects, followed by a trajectory with a progressive return to baseline over a maximum of 12 weeks, although the minimum sEMG and minimum force were not always recorded at the same time point. Three participants were able to complete only one to two segments. Slope values of the sEMG-force relations were also found to vary across the different time segments. While sEMG-force slopes provide assessments of force generation capacity of the BT injected muscle, amplitude histograms from novel sEMG recordings during the voluntary tasks provide additional insights about differential actions of BT on the overall motor unit (MU) population over time.Conclusions
The results of our study indicate that there are potential short term as well as long term decrements in muscle control and activation properties after BT administration on the affected side of chronic stroke survivors. Muscle activation levels as recorded using sEMG, did not routinely return to baseline even at three months’ post injection. The concurrent clinical measures also did not follow the same time course, nor did they provide the same resolution as our experimental measures. It follows that even 12 weeks after intramuscular BT injections muscle recovery may not be complete, and may thereby contribute to pre-existing paresis.Background
Spasticity
is a characteristic feature of upper motor neuron lesions, often
induced by a hemispheric stroke. The physical signs include hypertonia
(a perceived increase in resistance to externally imposed joint motion)
as well as hyper-reflexia, an increase in response to muscle stretch or
tendon tap. A potential contributor to hyper-reflexia in post-stroke
spasticity is hyper-excitability of the associated spinal motor neurons [1]. Spasticity is believed to affect up to 40% of the chronic stroke population [2].
Over the past decade, intramuscular BT is increasingly used in clinical
settings to temporarily reduce spasticity in a number of human
neurologic disorders [2,3,4],
including chronic stroke. Botulinum toxin is a metalloproteinase that
induces cholinergic blockade at the presynaptic level of the
neuromuscular junction. This chemo-denervation reduces hypertonia as
well as hyper-reflexia by reducing global motor outflow [5]. The cholinergic blockade fades away with time [6],
thus stroke survivors for whom BT is prescribed as part of their
clinical spasticity management plan often receive BT every 3–4 months [7].
Long term neuromuscular changes in muscles injected with BT have been
reported in animal studies, although, a systematic quantitative
evaluation of short and long-term neuromuscular consequences of BT on
injected muscles in humans has not been performed [8].
An initial reduction of voluntary muscle capacity is to be expected,
however, quantification of the initial reduction, as well as
quantification of the subsequent return to baseline values could provide
insight regarding neuromuscular recovery (the time course, voluntary
capacity level and neural control of muscle) subsequent to chemical
denervation. This information could be used to assist in driving
decisions about timing and dosage of repeated doses of BT as well as the
need for other rehabilitative therapies due to the potential of further
weakening paretic muscle. While BT is used to reduce spasticity, BT
broadly targets motor outflow so more extensive impairment of motor
function is possible.
Evaluations of BT mediated alterations in spasticity levels have largely been based on clinical assessments [9] which are at best semi-quantitative in nature, lack the necessary precision to track outcomes, and may not accurately describe the time course of BT effect on voluntary muscle activation, i.e., functional capacity. As a result, such clinical analyses do not fully address BT effects on voluntary muscle activity in the upper arm of injected stroke survivors. Several studies in humans have used joint torque as an alternative to assess both the short term and long term changes in functional capacity and neural control in various muscles after BT. Hameau et al. and Berunz et al. observed and successfully quantified the effect of BT effect on voluntary strength in lower limb in a cross-sectional study at four weeks post-injection [10, 11]. Additional electrophysiological studies were performed by Lee et al., who observed reduced values of the jaw muscle sEMG in healthy individuals at three weeks following intramuscular BT injections [12]. Hamjian and Walker reported only partial recovery of foot extensor muscle sEMG activity in healthy controls at successive intervals over the course of 3 months after the intramuscular BT in ten healthy controls based on ultrasound recordings as well as compound muscle action potential (CMAP) values [13]. A few studies have utilized electrophysiological techniques to assess changes in muscle function following BT injections in stroke survivors as well [14]. Albani et al. observed a reduction of sEMG values after BT injection in wrist flexor muscles of the stroke population at one and at 6 months post-injection [15]. Similar findings of reduced sEMG and force in biceps brachii muscle at one-month post-BT in stroke population were reported by Vinti et al. [16]. Hesse et al. found a reduction of sEMG in the soleus and tibialis anterior muscle after one month of injection amongst the majority of tested hemiparetic subjects [17]. Taken overall, these studies suggest that there are potential short-term as well as long term deficits during voluntary muscle activity following BT injections in stroke muscle. However, these studies were cross-sectional in nature and only one study provided data from both sEMG and force recordings of participants.
Accordingly, our global objective here is to use quantitative measures to accurately characterize the time course of changes in voluntary activation capacity of the biceps brachii on both sides of hemispheric stroke survivors who have received intramuscular BT on their affected side, as a part of their routine clinical care plan for spasticity. This assessment was accomplished in the usual time period between successive BT injections, 3–4 months, using force and sEMG recordings taken during varying voluntary isometric contractions. Based on the aforementioned studies, long term deficits in voluntary capacity could further weaken stroke survivors who routinely are diagnosed with paresis in addition to spasticity.
Furthermore, in related animal studies, several researchers reported BT chemo-denervation induced fiber atrophy and also a reduction in total alpha motor neuron numbers, in rats at two weeks post-injection [18, 19]. Subsequent to chemical denervation, Pamphlett reported early axonal sprouting within 2 days after the BT injection in mouse resulting from neuromuscular transmission failure [20] and this has been reported to have continued until 8 weeks by others [21]. In addition to affecting muscle contractile properties, BT injections also degrade muscle architecture, including sarcolemma structure, muscle stiffness, and muscle fiber density on histological examination, within four weeks [22]. Minamoto et al. reported reductions of joint torque by 50% after 6 months in rat tibialis anterior muscle and found that these changes were correlated with the altered muscle activity [23]. In addition, they reported a persisting sEMG deficit while the contraction force recovered after 12 weeks. Thus, unique short term and long term changes in neuronal control and muscle activity in response to intramuscular BT injections are evident in animal studies; these are also speculated but not reported in human studies. Thus we sought to address both the short term and long term changes in neural control and potential re-innervation with relevant recording and analysis techniques.
Specifically, we assessed the initial (expected) decline and subsequent return of both joint torque and sEMG values, and we determined whether both variables varied concurrently. In order to assess possible central effects, i.e., changes in muscle control in addition to peripheral alterations, we computed the slope of the relationship between the generated surface EMG and force during voluntary muscle activation, which has been shown to be disrupted in paretic stroke survivors [24]. We also sought to assess whether there were changes at the motor unit (MU) level, utilizing novel sEMG electrodes. By doing so, we are able to characterize changes in muscle activation capacity as well as assess any changes in the neural control of muscle.
To summarize our results, we found that there were systematic alterations of force and sEMG induced by BT even at 12 weeks post-injection, and we recorded consistent maximal reductions of the force and the sEMG between 2 and 4 weeks, consistent with results from animal studies. A part of this work was presented at Society for Neuroscience annual meeting, 2017.
Evaluations of BT mediated alterations in spasticity levels have largely been based on clinical assessments [9] which are at best semi-quantitative in nature, lack the necessary precision to track outcomes, and may not accurately describe the time course of BT effect on voluntary muscle activation, i.e., functional capacity. As a result, such clinical analyses do not fully address BT effects on voluntary muscle activity in the upper arm of injected stroke survivors. Several studies in humans have used joint torque as an alternative to assess both the short term and long term changes in functional capacity and neural control in various muscles after BT. Hameau et al. and Berunz et al. observed and successfully quantified the effect of BT effect on voluntary strength in lower limb in a cross-sectional study at four weeks post-injection [10, 11]. Additional electrophysiological studies were performed by Lee et al., who observed reduced values of the jaw muscle sEMG in healthy individuals at three weeks following intramuscular BT injections [12]. Hamjian and Walker reported only partial recovery of foot extensor muscle sEMG activity in healthy controls at successive intervals over the course of 3 months after the intramuscular BT in ten healthy controls based on ultrasound recordings as well as compound muscle action potential (CMAP) values [13]. A few studies have utilized electrophysiological techniques to assess changes in muscle function following BT injections in stroke survivors as well [14]. Albani et al. observed a reduction of sEMG values after BT injection in wrist flexor muscles of the stroke population at one and at 6 months post-injection [15]. Similar findings of reduced sEMG and force in biceps brachii muscle at one-month post-BT in stroke population were reported by Vinti et al. [16]. Hesse et al. found a reduction of sEMG in the soleus and tibialis anterior muscle after one month of injection amongst the majority of tested hemiparetic subjects [17]. Taken overall, these studies suggest that there are potential short-term as well as long term deficits during voluntary muscle activity following BT injections in stroke muscle. However, these studies were cross-sectional in nature and only one study provided data from both sEMG and force recordings of participants.
Accordingly, our global objective here is to use quantitative measures to accurately characterize the time course of changes in voluntary activation capacity of the biceps brachii on both sides of hemispheric stroke survivors who have received intramuscular BT on their affected side, as a part of their routine clinical care plan for spasticity. This assessment was accomplished in the usual time period between successive BT injections, 3–4 months, using force and sEMG recordings taken during varying voluntary isometric contractions. Based on the aforementioned studies, long term deficits in voluntary capacity could further weaken stroke survivors who routinely are diagnosed with paresis in addition to spasticity.
Furthermore, in related animal studies, several researchers reported BT chemo-denervation induced fiber atrophy and also a reduction in total alpha motor neuron numbers, in rats at two weeks post-injection [18, 19]. Subsequent to chemical denervation, Pamphlett reported early axonal sprouting within 2 days after the BT injection in mouse resulting from neuromuscular transmission failure [20] and this has been reported to have continued until 8 weeks by others [21]. In addition to affecting muscle contractile properties, BT injections also degrade muscle architecture, including sarcolemma structure, muscle stiffness, and muscle fiber density on histological examination, within four weeks [22]. Minamoto et al. reported reductions of joint torque by 50% after 6 months in rat tibialis anterior muscle and found that these changes were correlated with the altered muscle activity [23]. In addition, they reported a persisting sEMG deficit while the contraction force recovered after 12 weeks. Thus, unique short term and long term changes in neuronal control and muscle activity in response to intramuscular BT injections are evident in animal studies; these are also speculated but not reported in human studies. Thus we sought to address both the short term and long term changes in neural control and potential re-innervation with relevant recording and analysis techniques.
Specifically, we assessed the initial (expected) decline and subsequent return of both joint torque and sEMG values, and we determined whether both variables varied concurrently. In order to assess possible central effects, i.e., changes in muscle control in addition to peripheral alterations, we computed the slope of the relationship between the generated surface EMG and force during voluntary muscle activation, which has been shown to be disrupted in paretic stroke survivors [24]. We also sought to assess whether there were changes at the motor unit (MU) level, utilizing novel sEMG electrodes. By doing so, we are able to characterize changes in muscle activation capacity as well as assess any changes in the neural control of muscle.
To summarize our results, we found that there were systematic alterations of force and sEMG induced by BT even at 12 weeks post-injection, and we recorded consistent maximal reductions of the force and the sEMG between 2 and 4 weeks, consistent with results from animal studies. A part of this work was presented at Society for Neuroscience annual meeting, 2017.
Methods
Subject inclusion criteria
We have recruited and tested 8 hemiplegic stroke survivors with spasticity who were to receive botulinum toxin as part of their clinical care plan. The initial timing, dosage, and location of the injection were determined by their managing physician. All participants had sustained a single hemispheric stroke at least six months prior to experimental testing. Spasticity was assessed by a physical therapist using the Modified Ashworth Score (MAS) Subjects had to exhibit a score ≥ 1 to be included in the study. The subjects were included only if they were able to perform the required experimental tasks, attain the testing position of the hand, sit continuously during the experiments, and follow the instructions with intact auditory and visual perceptions. All participants gave informed consent via protocols approved by the Institutional Review Board under the Office for the Protection of Human Subjects at Northwestern University.Experimental setup
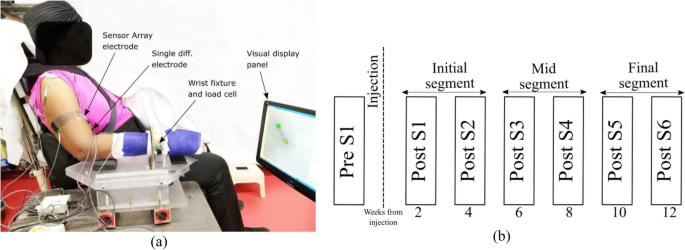
Participants were seated in a Biodex, Inc. chair with the forearm cast from elbow to the wrist and fixated to a ring-mount interface. The wrist mount was attached to a six-degrees-of-freedom force-torque sensor (ATI, Inc.: Delta ATI4007, Max force 660 N). After skin preparation, including a light skin abrasion and cleaning with alcohol pads, bipolar sEMG electrodes were placed on the medial and lateral heads of biceps brachii, triceps brachii and brachioradialis muscle (for online monitoring purposes). A pair of Delsys Inc., sensor array electrodes were mounted and aligned with the bipolar electrodes at a proximal location on the belly of the biceps muscle on both heads. The locations of electrodes were determined after muscle palpation on the belly of the muscle of interest, based on the recommendation of SENIAM [25]. The location of the electrodes and the load cell attachment was referenced to anatomical landmarks such as the bony prominence of the subject’s arm (lateral and medial epicondyles and acromion). SEMG recordings were performed during voluntary isometric, non-fatiguing elbow flexion force generation.A depiction of the experimental setup is shown in Fig. 1a. The EMG sensor locations were similarly maintained throughout all the sessions for all the subjects by measuring the electrode locations with respect to bony landmarks at the first pre-BT session and by documenting with photographs of the tested arm only. Signal acquisition was performed simultaneously through Spike 2® and the CED-1401®, and dEMG (Delsys Inc.) system decreasing force levels back to resting level. The force and the sEMG signal was sampled at a rate of 2 kHz.
Study protocol
Clinical assessments of spasticity, the Modified Ashworth Score (MAS), and motor impairment, the Fugl-Meyer assessment (FM) were performed by a research occupational therapist at each recording session on the upper extremity on both sides of our participants. After completing the experimental recording sessions on the affected side, patients were asked to return for testing on their contralateral limbs using the same protocol.Our protocol was designed to test the participants in one session before their respective BT injections (Pre-S1) and then 6 times after the injection (Post-S1- Post-S6), approximately every two weeks on both the affected and contralateral limbs. We have separated the observations in three segments across the time, initial segment (Post-S1-Post-S2), mid segment (Post-S3-Post-S4), and the final segment (Post-S5-PostS6) as shown in Fig. 1b. At each session, requisite submaximal force task levels were determined by asking the participants to perform maximum voluntary contractions during the session.
No comments:
Post a Comment