You'll want to know this if your doctor is trying to clean out plaque and clots in your peripheral arteries. You'll have to hope like hell your competent? doctor knows about this before you do.
Safety update: FDA announces Class I recall due to atherectomy devices breaking during use
The U.S. Food and Drug Administration (FDA) has provided an update on ongoing safety issues with atherectomy devices sold and distributed by Bard Peripheral Vascular, an Arizona-based subsidiary of Becton, Dickinson and Company (BD). When the agency first warned users about these issues back in February, it was still reviewing the situation; a recall had not yet been finalized.
With this update, however, the FDA has officially ruled that this is a Class I recall, meaning the devices “may cause serious injury or death” if used without following updated instructions for use (IFU) provided by Bard Peripheral Vascular.
The reason behind the new Class I recall
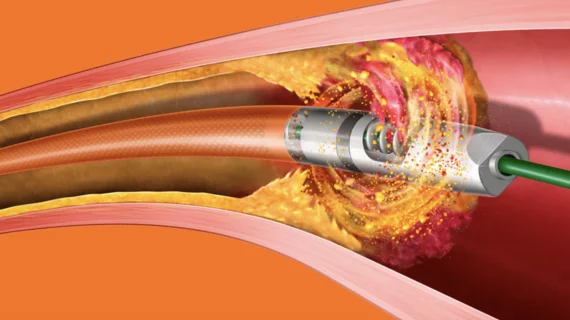
The warning includes multiple models of Bard Peripheral Vascular’s Rotarex Atherectomy System. These devices are designed to target high-risk plaques and blood clots in the peripheral arteries by rotating at a high speed. The ongoing issues have involved the helix portion of the devices fracturing or breaking off when being used.
“Use of the device in certain anatomy and lesion types, as well as certain procedural factors, may cause the helix to fracture or break, requiring retrieval of a broken catheter and/or device fragment,” according to the updated advisory. “A helix fracture or break could cause vessel injury and may lead to severe bleeding or death.”
This has resulted in 115 incidents that required an additional intervention. In addition, BD has received reports of 30 serious injuries and four deaths.
No devices need to be returned or removed from the market
Unlike many Class I recalls, where the devices are immediately removed from the market, clinicians can still use these atherectomy devices to treat patients. However, the FDA has emphasized that it is crucial to read the updated IFU before performing another procedure.
The updated IFU include several warnings, including:
- Use a kink resistant, suitably reinforced sheath of the same size as the Rotarex™ Atherectomy Catheter, or 1 French size bigger. When choosing a contralateral approach this may also serve to facilitate a smooth transition across the aortic bifurcation.
- Do not use the device across a vessel bifurcation or curve that results in a curvature of the catheter shaft of <4 cm in diameter (Figure 2). Consider the use of ipsilateral access if contralateral access is expected to result in a catheter bend less than 4 cm in diameter.
- Maintain adequate blood flow through the catheter to reduce the risk of catheter overheating or blockage. Adequacy of blood flow can be assessed by observing continuous drainage into the collecting bag and listening for changes in the audible pitch of the motor.
- Maintain constant catheter movement to reduce fatigue stress on the inner helix in one location. Perform a smooth back and forth motion within the target lesion. Use a 10 mm forward motion (equivalent to one catheter head) for softer materials and 1 mm for denser lesions.
- Do not use the device in calcified vessel segments that exhibit radiopacities on both sides of the arterial wall and extend beyond 10 mm in length prior to contrast injection or digital subtraction angiography.
- Monitor the catheter closely for resistance to movement. Audible control unit alarms (i.e., intermittent beeping) or changes in tactile feel of the catheter, pitch sound of the motor, or LED bar illumination on the control unit (where the green light is no longer illuminated, leaving only the yellow/orange light illuminated) indicate the need to reduce catheter advancement from increments of 10 mm to 1 mm, stop, or flush the catheter.
Click here for more information from the FDA and Bard Peripheral Vascular.
No comments:
Post a Comment