It seems apparent to me(not medically trained) that brain integration is needed post stroke and post TBI. But I bet your doctor and hospital did nothing with psilocybin and micro-dosing LSD since the earliest research came out.
THIS IS STRICTLY FOR YOUR DOCTOR TO ANSWER. Can this help stroke recovery? And if your doctor incompetently does not know the answer, fire them. Ask your doctor why psilocybin hasn't already been prescribed.
psilocybin (14 posts to May 2014)
LSD (3 posts to September 2018 )
magic mushrooms (17 posts to October 2014)
The latest here; why is your hospital so fucking incompetent they haven't explored these solutions?
Increased global integration in the brain after psilocybin therapy for depression
Nature Medicine (2022)
Abstract
Psilocybin therapy shows antidepressant potential, but its therapeutic actions are not well understood. We assessed the subacute impact of psilocybin on brain function in two clinical trials of depression. The first was an open-label trial of orally administered psilocybin (10 mg and 25 mg, 7 d apart) in patients with treatment-resistant depression. Functional magnetic resonance imaging (fMRI) was recorded at baseline and 1 d after the 25-mg dose. Beck’s depression inventory was the primary outcome measure (MR/J00460X/1). The second trial was a double-blind phase II randomized controlled trial comparing psilocybin therapy with escitalopram. Patients with major depressive disorder received either 2 × 25 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily placebo (‘psilocybin arm’) or 2 × 1 mg oral psilocybin, 3 weeks apart, plus 6 weeks of daily escitalopram (10–20 mg) (‘escitalopram arm’). fMRI was recorded at baseline and 3 weeks after the second psilocybin dose (NCT03429075). In both trials, the antidepressant response to psilocybin was rapid, sustained and correlated with decreases in fMRI brain network modularity, implying that psilocybin’s antidepressant action may depend on a global increase in brain network integration. Network cartography analyses indicated that 5-HT2A receptor-rich higher-order functional networks became more functionally interconnected and flexible after psilocybin treatment. The antidepressant response to escitalopram was milder and no changes in brain network organization were observed. Consistent efficacy-related brain changes, correlating with robust antidepressant effects across two studies, suggest an antidepressant mechanism for psilocybin therapy: global increases in brain network integration.
Main
Depression is a highly prevalent mental health condition1, the incidence of which has increased during the COVID-19 pandemic2, for example, as reflected in increased prescriptions of antidepressant medications3. However, even the best-performing antidepressant drugs show modest efficacy, non-negligible side effects, discontinuation problems and high relapse rates4,5,6,7, highlighting the need for new, improved treatments8.
Patients with a diagnosis of depression often exhibit a negative cognitive bias, characterized by pessimism, poor cognitive flexibility, rigid thought patterns and negative fixations regarding ‘self’ and the future9,10. A number of authors have directly or indirectly taken inspiration from dynamical systems theory to describe depressive episodes as ‘attractor states’ (stereotyped cognitive states with ‘gravitational pull’11).
Neuroimaging research has consistently found examples of abnormal brain functioning in depression, resonant with its phenomenology12,13,14. A hierarchically supraordinate intrinsic brain network15, the default mode network (DMN), is associated with introspection and self-referential thinking16. These cognitive functions are often overactive in depression9, and several studies have linked excessive engagement of DMN functioning with depressive symptomatology12.
In addition to the DMN, other higher-order brain networks such as the executive network (EN) and salience network (SN) have been implicated in depression14,17. These networks are associated with ‘cognitive control’ and internal versus external attention switching18,19,20. Such attentional switching is often impaired in depression21. Tellingly, the serotonin 2A (5-HT2A) receptor subtype, which is the key proteomic binding site of ‘classic’ serotonergic psychedelic drugs, such as psilocybin22, is most densely expressed in a broad pattern of cortex that closely resembles a conjunction map of the DMN, EN and SN23, corresponding to the transmodal portion of the brain’s principal hierarchical gradient15.
In the last 15 years, at least six separate clinical trials have reported impressive improvements in depressive symptoms with psilocybin therapy24. Included among these studies are (1) an open-label trial in treatment-resistant depression25 and (2) a double-blind, randomized controlled trial (DB-RCT) with an active comparator, the selective serotonin reuptake inhibitor (SSRI) and conventional antidepressant, escitalopram26. These two trials, which included pre-treatment and post-treatment fMRI, are the focus of this paper’s analyses.
The therapeutic action of psilocybin and related psychedelics is incompletely understood; however, one model proposes that psychedelics cause a 5-HT2A receptor-induced dysregulation of spontaneous population-level neuronal activity, linked to a temporary ‘disintegration’ of intrinsic functional brain networks27 and a hypothesized decrease in the precision-weighting of predictive models encoded (at least in part) by the integrity of functional modules28. One important corollary of modular ‘disintegration’ seems to be the broadening of the brain’s functional repertoire of states, commensurate with a broader or flatter global energy landscape29.
Here we hypothesize that the well-replicated finding of brain network disintegration and desegregation under psychedelics30,31 will be apparent subacutely, in post-treatment resting-state fMRI data. We also hypothesize that this effect, consistent with a flatter energy landscape, will relate to improved depression outcomes and will not be observed after a course of the SSRI, escitalopram.
Results
Open-label trial
Rapid antidepressant effect of psilocybin therapy
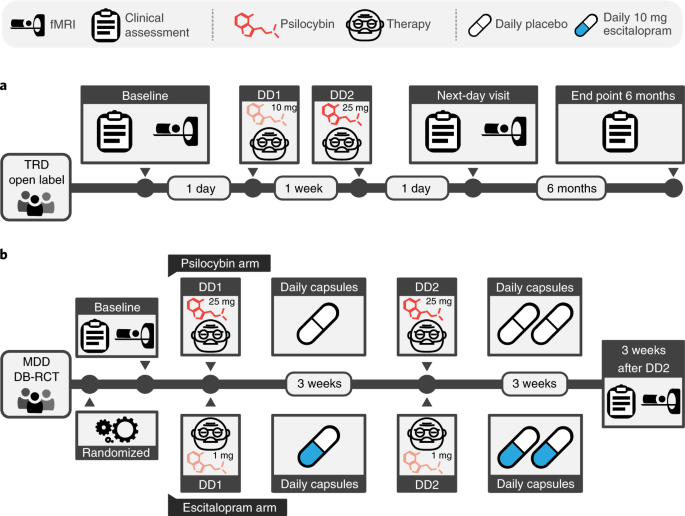
Patients with treatment-resistant depression (TRD) participated in a single-arm, open-label psilocybin therapy clinical trial (Fig. 1a). Baseline clinical assessment and resting-state fMRI were followed by fixed-order ‘low’ (10 mg) and ‘high’ (25 mg) psilocybin therapy dosing days (DDs) that were separated by 1 week. A second clinical assessment and fMRI scan were conducted 1 d after DD2. Remote assessments of clinical status were conducted 1 week, 3 months and 6 months after DD2. Further details are available in Methods and elsewhere25.
a, Open-label trial. Eligible patients attended a baseline clinical assessment and resting-state fMRI visit. This was followed by two orally administered psilocybin therapy DDs separated by 1 week, which differed in dose strength (10 mg on DD1, 25 mg on DD2). The post-treatment fMRI scan occurred 1 d after DD2. Remote clinical assessment continued for 6 months. b, DB-RCT. Patients attended a baseline clinical assessment and resting-state fMRI visit and were randomly assigned to the psilocybin arm (top) or escitalopram arm (bottom). The psilocybin arm involved 2 × 25 mg psilocybin therapy DDs with 3 weeks of daily placebo capsules following each DD. The escitalopram arm involved 2 × 1 mg psilocybin therapy DDs with 3 weeks of 10 mg daily escitalopram following DD1 and 20 mg of escitalopram following DD2. Both groups attended a post-treatment clinical assessment and fMRI visit 3 weeks and 1 d after DD2.
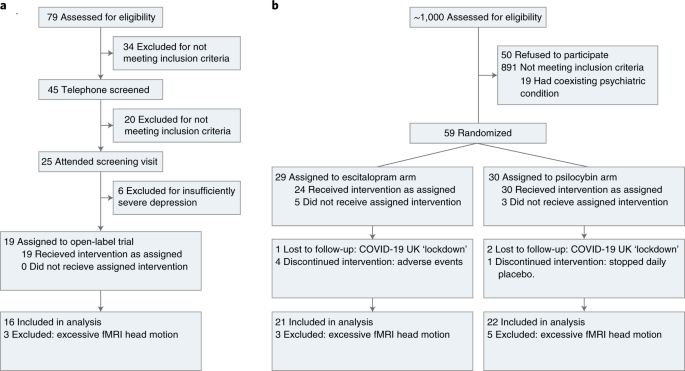
Of the 19 patients recruited, 3 were excluded due to excessive fMRI head motion (Fig. 2a). We first confirmed an antidepressant effect of psilocybin in this imaging sample of 16 patients (mean age, 42.75 years, s.d. = 10.15, 4 females) using the Beck Depression Inventory (BDI-1A). This patient-rated measure was preregistered for the original investigation (gtr.ukri.org MR/J00460X/1). The BDI captures a broad range of symptoms and places particular emphasis on the cognitive features of depression32, which may be an important target of psilocybin therapy.
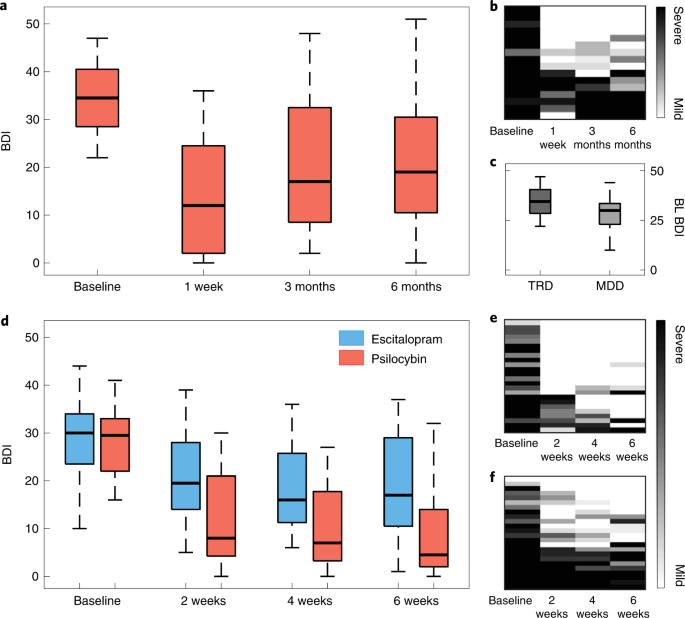
Baseline BDI scores indicated severe depression (mean BDI = 34.81, s.d. = 7.38). In line with our previous report25, rapid, substantial and sustained reductions in depression severity were observed after treatment (Fig. 3a,b). Relative to baseline, significant BDI reductions were observed at 1 week (mean difference, −21.0 points; t15 = 7.11, 95% confidence interval (CI) −27.30 to −14.71, P < 0.001, Cohen’s d = 1.78) and still evident at 6 months (mean difference, −14.19 points; t15 = 4.26, 95% CI −21.29 to −7.09, P < 0.001, d = 1.07).
a, Open-label trial box plots of BDI scores of patients with TRD across time points. b, Qualitative raster plots of individual patient’s BDI score for each time point (columns). c, Baseline BDI of patients with TRD in the open-label trial was significantly greater than that in patients with MDD in the DB-RCT (t57 = 3.01, 95% CI 2.18 to 10.88, P = 0.013, d = 0.83). d, DB-RCT BDI scores from each study arm and time point. e,f, Qualitative raster plots of individual patient’s BDI for each time point for the psilocybin arm (e) and escitalopram arm (f). The box plot central marks represent the group median, the box edges represent the 25th to 75th percentiles and the whiskers extend to the data range. Independent samples of n = 16 TRD were used in a and alongside n = 43 MDD in c, of which n = 21 in the escitalopram arm and n = 22 in the psilocybin arm are displayed in d. The rows from each qualitative raster plot were ordered by the sum BDI score across time points.
Decreased brain modularity one day after psilocybin therapy
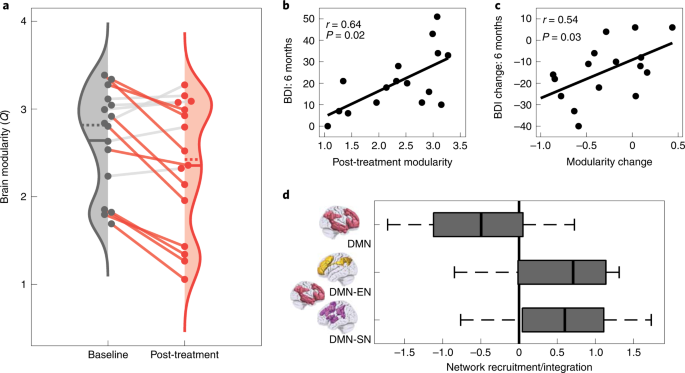
To test our primary hypothesis, preprocessed fMRI data were used to estimate normalized network modularity from Pearson correlation functional connectivity matrices of the cortex (Methods). Confirming our primary hypothesis, brain network modularity was significantly reduced (Fig. 4a) 1 d after psilocybin therapy (mean difference, −0.29; t15 = 2.87, 95% CI 0.07 to 0.50, P = 0.012, d = 0.72). This result implies a global increase in functional connectivity between the brain’s main intrinsic networks.
a, Brain modularity (normalized Q) significantly reduced, indicating a global increase in brain network integration following psilocybin therapy in patients with TRD (t15 = 2.87, 95% CI 0.07 to 0.50, P = 0.012, d = 0.72). The solid and dotted lines represent the mean and median, respectively. Patient’s data are connected by solid lines and rendered in color if modularity decreased. b, Absolute post-treatment scan modularity correlated with absolute BDI scores at the 6 months primary end point (r14 = 0.64, 95% CI 0.29 to 0.84, P = 0.023, FDR-corrected). c, Post-treatment change in brain modularity significantly correlated with treatment response (BDI, baseline − 6 months) (r14 = 0.54, 95% CI 0.14 to 0.78, P = 0.033). d, DMN (red) recruitment decreased (t15 = −2.99, 95% CI −0.92 to −0.15, P = 0.009, d = 0.75) and its between-network integration with the EN (gold) (t15 = 3.01, 95% CI 0.15 to 0.90, P = 0.01, d = 0.75) and SN (purple) (t15 = 2.89, 95% CI 0.14 to 0.95, P = 0.01, d = 0.72) increased following psilocybin therapy (all FDR-corrected). The box plot central marks represent the group median, the box edges represent the 25th to 75th percentiles and the whiskers extend to the data range. Independent samples of n = 16 TRD were used in a–d.
Decreased modularity predicts improved clinical outcomes
We hypothesized that decreased brain network modularity would relate to the sustained improvements in depression severity that follow psilocybin therapy. To test this, we calculated Pearson correlations between the post-treatment brain modularity and BDI scores from the three post-treatment time points (1 week, 3 months, 6 months). After false discovery rate (FDR) correction for multiple comparisons, a strong significant Pearson correlation was observed at the 6 months primary end point (r14 = 0.64, 95% CI 0.29 to 0.84, P = 0.023; Fig. 4b). Directionally consistent relationships were seen at 3 months (r14 = 0.46, 95% CI 0.03 to 0.74, P = 0.114) and 1 week (r14 = 0.29, 95% CI −0.16 to 0.64, P = 0.284), but these did not survive correction. Pre-treatment versus post-treatment changes in modularity significantly correlated with change in BDI score at 6 months, relative to baseline (r14 = 0.54, 95% CI 0.14 to 0.78, P = 0.033; Fig. 4c). These results imply that decreased brain modularity 1 d after psilocybin therapy relates to long-term improvements in depression symptom severity.
DMN changes in within-network and between-network functional connectivity one day after treatment
Previous research has implicated depressive symptomology with hyperconnectivity of the DMN12 and hypoconnectivity of the DMN with other higher-order ‘cognitive’ networks, including the EN and SN14,17. We therefore tested for evidence of these abnormalities being attenuated after treatment using functional cartography (Methods). Consistent with our previous hypothesis, significant reductions in DMN network recruitment (mean difference, −0.54; t15 = −2.99, 95% CI −0.92 to −0.15, P = 0.009, d = 0.75; Fig. 4d) and increased between-network integration between the DMN and EN (mean difference, 0.53; t15 = 3.01, 95% CI 0.15 to 0.90, P = 0.01, d = 0.75) and between the DMN and SN (mean difference, 0.55; t15 = 2.89, 95% CI 0.14 to 0.95, P = 0.01, d = 0.72; FDR-corrected) were observed 1 d after psilocybin therapy. An exploratory analysis of the changes in network recruitment and between-network integration of other brain networks is available in Supplementary Fig. 2.
Taken together, these findings indicate a clinically relevant decrease in brain network modularity following psilocybin therapy for TRD. A network cartography analysis suggests that this global change in network organization may be underpinned by a specific decrease in within-DMN connectivity and increase in DMN connectivity with other higher-order networks, including the EN and SN.
Double-blind randomized controlled trial
Psilocybin therapy versus escitalopram for depression
The design of this DB-RCT (Fig. 1b) gave a new opportunity to compare not just the safety and efficacy, but also the mechanisms of action of psilocybin therapy to those of a conventional antidepressant drug, escitalopram. Patients with major depressive disorder (MDD) were randomly allocated to a ‘psilocybin arm’ or ‘escitalopram arm’ (Fig. 1b). Baseline clinical assessment and resting-state fMRI was followed by DD1, when patients received either 25 mg psilocybin (psilocybin arm) or a presumed inactive 1 mg psilocybin dose (escitalopram arm). All patients were informed that they would receive psilocybin but were blind to the dosage. DD2 occurred 3 weeks after DD1 and was a duplicate dosage. Beginning 1 d after DD1, patients took daily capsules for 6 weeks and 1 d in total. For both conditions, one capsule per day was ingested for the first 3 weeks and two capsules per day were ingested thereafter. Capsule content was either inert placebo (microcrystalline cellulose in the psilocybin arm) or 10 mg escitalopram in the escitalopram arm (10 mg daily for the first 3 weeks and 2 × 10 mg (20 mg) daily for the final 22 d). Further details are available in Methods and elsewhere26.
Of the 59 patients with MDD recruited, 29 were randomly allocated to the escitalopram arm. Of those, four discontinued due to adverse reactions to escitalopram, one was lost due to the COVID-19 UK lockdown and three were excluded due to excessive fMRI head motion (Fig. 2b). The remaining 21 patients (mean age, 40.9 years, s.d. = 10.1, 6 female) were included in the escitalopram imaging sample. Thirty patients were randomly allocated to the psilocybin arm. Of those, one was excluded for choosing not to take the daily (placebo) capsules, two did not attend the post-treatment session due to the COVID-19 UK lockdown and five were excluded due to excessive fMRI head motion. The remaining 22 patients (mean age, 44.5 years, s.d. = 11.0, 8 female) were included in the psilocybin imaging sample (Fig. 2b).
The BDI was a primary outcome measure for the open-label trial (MR/J00440/1) and a secondary outcome measure for this DB-RCT (ClinicalTrials.gov identifier NCT03429075); however, this measure proved to be an especially sensitive index of post-psilocybin reductions in symptom severity across the trials26. Between trials, baseline BDI (Fig. 3c) was significantly greater in the open-label TRD trial compared with the DB-RCT MDD trial (mean difference, 6.53 points; t57 = 3.01, 95% CI 2.18 to 10.88, P = .013, d = 0.83). This difference is likely due to TRD being an inclusion criteria in the open-label trial, but not in this DB-RCT.
As described in our previous report26, BDI-measured reductions in depressive symptom severity were significantly greater after psilocybin than escitalopram, indicating superior efficacy of psilocybin therapy versus escitalopram (Fig. 3d). Moreover, we confirmed the statistical significance of this contrast within the smaller neuroimaging sample included in the present analyses after testing for an arm × time point analysis of variance interaction on BDI scores (F3,123, 4.47; P = 0.005). FDR-corrected pairwise comparisons relative to baseline were significantly different at 2 weeks (mean difference, −8.73; t41 = −3.66, 95% CI −13.55 to −3.91, P = 0.002, d = 0.98), 4 weeks (mean difference, −7.79; t41 = −2.69, 95% CI −13.62 to −1.95, P = 0.013, d = 0.77) and at 6 weeks (mean difference, −8.78; t41 = −2.61, 95% CI = −15.58 to −1.97, P = 0.013, d = 0.75), all favoring the psilocybin arm.
More at link.
No comments:
Post a Comment