This moon shot is the best use of following the stroke strategy to get everyone 100% recovered? I don't think so.
But I'd rather they tackle known problems first. Like research that stops the 5 causes of the neuronal cascade of death in the first week. Much more likely to succeed.
Adult Stem Cells and Induced Pluripotent Stem Cells for Stroke Treatment
- Clinical Neuroscience Research Laboratory, Health Research Institute of Santiago de Compostela (IDIS), Santiago de Compostela, Spain
Introduction
From the moment that the capacity of differentiation and
self-renewal of stem cells became known, their use as cell therapy for a
wide range of diseases has been considered. The international community
has focused on this idea, starting a revolution in the study of stem
cells (1).
This revolution led to several important discoveries that, step by
step, paved the way to convert cell therapy into reality. But the
greatest discovery was made in 2006 when Yamanaka and Takahashi were
able, for the first time, to generate induced pluripotent stem cells
(iPSCs) from adult somatic cells by inducing the artificial expression
of four transcriptional factors: OCT4, SOX2, c-MYC, and KLF4 (2).
This new approach provided a considerable resource of human pluripotent
stem cells that could be propagated during long-term culture and yet be
differentiated to a variety of lineages representatives of the three
embryonic germ layers, solving the ethical limitations caused by the use
of human embryonic stem cells.
In addition, the generation of human iPSCs from different
somatic cells of patients and the subsequent differentiation to the
affected cell lineage has allowed the recapitulation of features of
genetic pathologies through in vitro disease modeling and the
discovery of new treatments directly tested on these human cells.
Recently, the combination of iPSCs with the advances in genome editing
techniques, such as the clustered regularly interspaced short
palindromic repeat (CRISPR) system, has also provided a promising way to
repair putative causative alleles in patient lines into a healthy cell
line for future autologous cell therapy (3, 4) (Figure 1).
FIGURE 1
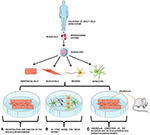 Figure 1. iPSCs modeling scheme. Adult somatic cells
(e.g., blood cells) are collected from the patient, reprogrammed and
derived to the affected cell types (e.g., endothelial cells, muscle
cells, neurons, or astrocytes), which are co-cultured in vitro,
opening the possibility to perform several studies directly on the
patient's own cells. Adapted from Servier Medical Art by Servier is
licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com/).
Figure 1. iPSCs modeling scheme. Adult somatic cells
(e.g., blood cells) are collected from the patient, reprogrammed and
derived to the affected cell types (e.g., endothelial cells, muscle
cells, neurons, or astrocytes), which are co-cultured in vitro,
opening the possibility to perform several studies directly on the
patient's own cells. Adapted from Servier Medical Art by Servier is
licensed under a Creative Commons Attribution 3.0 Unported License (https://smart.servier.com/).
The development of human iPSCs
has also opened a new opportunity for those neurological diseases where
the affected neuronal type is well-known or the genetic cause of the
pathology is well-described such as (i) Alzheimer's (5, 6), (ii) Parkinson's (7, 8), (iii) amyotrophic lateral sclerosis (9), or (iv) Huntington disease (10).
In these pathologies, iPSCs have been used to generate neuronal cell
lines to recapitulate and study the mechanics of the pathology in in vitro models or to evaluate their neurorecovery capability.
In the field of stroke, like other stem cells, iPSCs have
been used as a neuroprotective cell therapy (mainly based on their
immunomodulatory capacity) or as a neuroreparative therapy (by inducing
neurogenesis, angiogenesis, synaptogenesis, modulation of the immune
response, or transdifferentiation) (Figure 2).
Besides its neuroprotective or neuroreparative application, the use of
iPSCs for stroke modeling has been poorly exploited mainly because this
is a neurological pathology with multiple affected cells types and
reduced genetic component, compared to other neurological diseases such
as Alzheimer's or Parkinson's. However, the use of iPSCs has been
recently explored to model neurovascular pathologies associated with
risk of stroke (11, 12), opening a promising approach in the study of these neurovascular diseases.
FIGURE 2
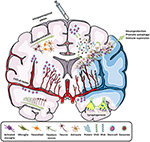 Figure 2. Scheme of all the main effects promoted by
stem cells in stroke. By intraparenchymal injection or i.v./i.a.
routes, stem cells induce neurogenesis, transdifferentiation,
angiogenesis, synaptogenesis, and immune modulation by attracting or
releasing trophic substances to the infarcted area. Adapted from Servier
Medical Art by Servier is licensed under a Creative Commons Attribution
3.0 Unported License (https://smart.servier.com/).
Figure 2. Scheme of all the main effects promoted by
stem cells in stroke. By intraparenchymal injection or i.v./i.a.
routes, stem cells induce neurogenesis, transdifferentiation,
angiogenesis, synaptogenesis, and immune modulation by attracting or
releasing trophic substances to the infarcted area. Adapted from Servier
Medical Art by Servier is licensed under a Creative Commons Attribution
3.0 Unported License (https://smart.servier.com/).
In this review, we offer a general
overview of the use of adult stem cells and iPSCs in stroke, addressing
the main problems and the main clinical trials that already present
results.
Adult Stem Cell Therapy in Stroke
Stroke, resulting from the interruption of blood supply
to the brain, is the leading cause of disability and death in the world
within neurological diseases despite a decrease in its mortality rate (13).
Pharmacological or mechanical reperfusion therapies are the most
effective treatments during the acute phase of ischemic stroke and it is
associated with good outcome in 50–70% of cases. However, these
treatments are only applicable to <20% of patients because of the
short therapeutic window and side effects (14).
Stem-cell-based therapies have emerged as a promising
tool for the treatment of both acute and delayed phases of stroke owing
to their multipotentiality, ability to release growth factors, and
immunomodulatory capacities. Thus, this transdifferentiation is able to
produce cells with a neural lineage; induce neurogenesis, angiogenesis,
and synaptogenesis; and activate endogenous restorative processes
through the production of cytokines and trophic factors. Moreover, the
regulation of cerebral blood flow (CBF), the blood–brain barrier (BBB),
and other neuroprotective mechanisms, such as the reduction of
apoptosis, inflammation, and demyelination or the increase of astrocyte
survival, have also been described as beneficial after stroke (15).
While the technology of the iPSCs is quite new and deeper
studies are being carried out to know its real translationality,
studies with adult stem cells have been performed for much longer, and
there is more information about their use in cell therapy for stroke.
Furthermore, there are already clinical trials going on and even closed
with adult stem cells. Focusing on stroke, the most frequently used stem
cells are the mesenchymal stem cells (MSCs), due to their great trophic
capabilities, and the neural stem cells (NSCs), because of their
neurorecovery activity (15).
More at link.
 Héctor Fernández-Susavila,
Héctor Fernández-Susavila,  Francisco Campos
Francisco Campos
No comments:
Post a Comment