Interesting discussion but I got nothing out of it that is going to help recovery.
Motor Overflow and Spasticity in Chronic Stroke Share a Common Pathophysiological Process: Analysis of Within-Limb and Between-Limb EMG-EMG Coherence
- 1Department of Physical Medicine and Rehabilitation, McGovern Medical School, University of Texas Health Science Center – Houston, Houston, TX, United States
- 2TIRR Research Center, TIRR Memorial Hermann Hospital, Houston, TX, United States
The phenomenon of exaggerated motor overflow is well documented in stroke survivors with spasticity. However, the mechanism underlying the abnormal motor overflow remains unclear. In this study, we aimed to investigate the possible mechanisms behind abnormal motor overflow and its possible relations with post-stroke spasticity. 11 stroke patients (63.6 ± 6.4 yrs; 4 women) and 11 healthy subjects (31.18 ± 6.18 yrs; 2 women) were recruited. All of them were asked to perform unilateral isometric elbow flexion at submaximal levels (10, 30, and 60% of maximum voluntary contraction). Electromyogram (EMG) was measured from the contracting biceps (iBiceps) muscle and resting contralateral biceps (cBiceps), ipsilateral flexor digitorum superficialis (iFDS), and contralateral FDS (cFDS) muscles. Motor overflow was quantified as the normalized EMG of the resting muscles. The severity of motor impairment was quantified through reflex torque (spasticity) and weakness. EMG-EMG coherence was calculated between the contracting muscle and each of the resting muscles. During elbow flexion on the impaired side, stroke subjects exhibited significant higher motor overflow to the iFDS muscle compared with healthy subjects (ipsilateral or intralimb motor overflow). Stroke subjects exhibited significantly higher motor overflow to the contralateral spastic muscles (cBiceps and cFDS) during elbow flexion on the non-impaired side (contralateral or interlimb motor overflow), compared with healthy subjects. Moreover, there was significantly high EMG-EMG coherence in the alpha band (6–12 Hz) between the contracting muscle and all other resting muscles during elbow flexion on the non-impaired side. Our results of diffuse ipsilateral and contralateral motor overflow with EMG-EMG coherence in the alpha band suggest subcortical origins of motor overflow. Furthermore, correlation between contralateral motor overflow to contralateral spastic elbow and finger flexors and their spasticity was consistently at moderate to high levels. A high correlation suggests that diffuse motor overflow to the impaired side and spasticity likely share a common pathophysiological process. Possible mechanisms are discussed.
Introduction
When a stroke survivor with spastic hemiplegia is asked to squeeze the hand or flex the elbow joint on the non-impaired side as shown in Figure 1, there is involuntary activation of spastic finger and elbow flexors on the impaired side (Figures 1A, B). This phenomenon of involuntary activation of spastic muscles can occur in about 30% of hemiplegic stroke (1). It is often referred as motor overflow or associated reaction (1–8). Other terms, such as mirror movement, global synkinesis, are sometimes used interchangeably for the same clinical observation (8). Motor overflow is one form of the spastic muscle overactivity. Other types of muscle overactivity are also seen clinically, such as spastic dystonia, co-contraction (9, 10).
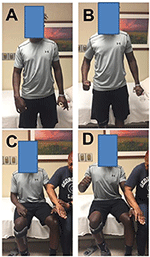
Figure 1. Motor overflow in a 41 year old stroke survivor with right spastic hemiplegia from a left middle cerebral artery hemorrhagic stroke. (A) standing and relaxed; (B) standing and left hand squeezing; (C) sitting and relaxed; (D) sitting and resisted hand/finger extension on the left side. Photos were recently taken from PI's spasticity clinic, a written consent of media release was signed by the patient.
Motor overflow is commonly observed in the contralateral homologous resting muscle(s). It can also be seen from proximal muscles to distal muscles in a form of abnormal synergy (11, 12), and between limbs on the impaired side through interlimb coupling (13). As demonstrated in Figures 1C,D, motor overflow to the contralateral spastic finger and elbow flexors occurs during voluntary finger extension on the non-impaired side. These clinical presentations indicate that motor overflow to the spastic muscles is non-selective, diffuse, and concomitantly with voluntary activation of other muscles. In contrast, motor overflow seen in neurologically intact adults is mainly in contralateral homologous muscles in the context of extreme effort or fatigue [see review (14)]. Therefore, motor overflow in stroke survivors is likely mediated by different mechanisms than in healthy adults. However, the underlying mechanisms for motor overflow after stroke are poorly understood.
A number of methods have been used in the literature to evaluate motor overflow after neurological impairments, including surface EMG, goniometry, dynamometry, electrogoniometry, and clinician rating form. Surface EMG is the most commonly used laboratory-based method (8). In our recent studies (15, 16), involuntary EMG activities of the contralateral resting muscles were used to quantify the extent of motor overflow during unilateral voluntary elbow flexion tasks. Using quantitative assessment, the level of motor overflow is found to be graded by the effort of the non-impaired muscles (3). Furthermore, EMG-EMG coherence analysis between EMG signals from the contracting muscle and the contralateral resting muscles could provide potential sources of motor overflow. Coherence analysis is based on the cross-correlation between two separate signals in the frequency domain. Coherence values fall between 0 and 1. Commonly studied frequency bands include 6–12 Hz (alpha band), 13–30 Hz (beta band), and 30–60 Hz (gamma band). It is well accepted that both beta and gamma bands have cortical origins (17–20). Coherence in the alpha band is believed to have subcortical influences, may be related to the reticulospinal drive (21). For example, EMG signals were recorded from bilateral homologous muscles, such as biceps muscles during motoric responses of acoustic startle reflex and during similar voluntary movements in healthy subjects. EMG-EMG coherence in the alpha band was significantly greater during startle reflex responses than during voluntary movement, suggestive of a reticulospinal origin of such coherence in the alpha band (21).
Motor overflow is often seen and elicited in stroke survivors with spasticity. Its relation with post-stroke spasticity remains controversial. Motor overflow is found to be associated with spasticity in some studies (2, 3, 6), but not in others (1, 4). In all these studies, spasticity was assessed using clinical scales, such as modified Ashworth scale or Tardieu scale. Quantitative assessment is likely to provide better insights into this relationship. Based on the velocity-dependent increase in resistance feature of spasticity, a quantitative assessment with computerized control of external stretch was developed (22, 23). In this approach, a joint is stretched by a motorized device at a controlled, constant speed. Resistance torque is obtained to quantify responses from spastic muscles. Reflex torque is quantified objectively by subtracting passive resistance at a very slow speed of stretch, e.g., 5°/s from that at a fast speed, e.g., 100°/s. Reflex torque is attributed primarily to underlying neural mechanisms of spasticity. In a previous study (24), we have demonstrated that reflex torque was velocity-dependent at the same wrist position (muscle length), and changed with various wrist positions at the same speed of stretch. This biomechanical quantification of spasticity is also sensitive to quantify reflex and non-reflex responses from spastic elbow flexors in response to controlled cold exposure (25).
In the present study, the specific aim was to examine the possible mechanisms mediating the phenomenon of motor overflow in chronic stroke. Stroke survivors and healthy controls were instructed to flex the elbow joint voluntarily at submaximal levels. Surface EMG signals were recorded from bilateral elbow flexors and finger flexors to quantify motor overflow. Within-limb and between-limb EMG-EMG coherence analyses were performed. Elbow flexor spasticity was quantified using our established biomechanical approach. Since motor overflow is commonly seen in stroke survivors with spasticity, they may share the same underlying pathophysiology. We hypothesized that there is greater motor overflow to the spastic elbow and finger flexors and that greater motor overflow is highly correlated with spasticity, as compared to the control group. Furthermore, post-stroke spasticity is primarily attributed to reticulospinal hyperexcitability and has separate underlying mechanisms for weakness (26, 27). between-limb intermuscular EMG signals were hypothesized to have significant EMG-EMG coherence in the alpha band to reflect reticulospinal hyperexcitability. Motor overflow was further hypothesized to correlate with spasticity (reflex torque), but not weakness.
 Yen-Ting Chen
Yen-Ting Chen Shengai Li
Shengai Li Elaine Magat1,2,
Elaine Magat1,2,  Ping Zhou
Ping Zhou Sheng Li
Sheng Li
No comments:
Post a Comment