This is absolutely appalling that they think 'care' is what survivors want. YOU FUCKING BLITHERING IDIOTS; they want 100% recovery, nothing less. GET THERE!
Device design and development tips for the future of stroke care

Michael Gilvarry is the GM of Cerenovus in Galway, Ireland. [Photo courtesy of Johnson & Johnson MedTech]
That’s according to Michael Gilvarry, GM of Johnson & Johnson MedTech’s Cerenovus Galway business. While he declined to divulge his neurovascular unit’s plans for future products, he offered advice for other device developers in a field where every minute counts.
A large vessel ischemic stroke can destroy more than 2 million brain neurons per minute before treatment restores oxygenated blood flow. A study quantifying the “time is brain” stroke mantra found that hourly neuron loss is equivalent to more than three years of normal aging.
“The drive for simplicity in stroke is very, very strong,” Gilvarry said in a Medical Design & Outsourcing interview. “It’s not like an elective procedure where you can plug something in, look at settings, get feedback. Stroke is really about getting that clot out as quickly as possible. … Simplicity is going to be super important in the evolution of stroke treatment.”
Simpler stroke devices will not only help doctors remove clots faster, but they’ll offer more options for patients globally who don’t live near stroke centers staffed by experienced neurosurgeons.
Imaging innovation can accelerate stroke treatment
Before treating a stroke patient, healthcare providers need to diagnose the stroke and determine whether it’s ischemic or hemorrhagic. Of the approximately 800,000 strokes each year in the U.S., nearly 90% are ischemic strokes caused by a clot rather than a leaking blood vessel or ruptured aneurysm.
Ischemic strokes can often be treated with clot-dissolving tissue plasminogen activator (tPA) medicine, but those “clot-busters” will only make things worse for a hemorrhagic stroke patient with a brain bleed.
Advances in imaging technology such as computed tomography (CT) scans in ambulances can help get ischemic stroke patients started on tPA to restore blood flow sooner. These specialized mobile stroke units can administer tPA immediately and share scans with neurosurgeons at the hospital before their patient arrives.
“Getting the patient onto the table is a challenge because sometimes these patients are sitting in emergency departments for long periods of time before it’s even diagnosed as a stroke through imaging,” Gilvarry said. “And when it comes to the interventional part of it, if a physician could know in advance exactly what they’re dealing with in terms of clot location and clot composition, it might change their approach.”
Fast, first-pass clot removal
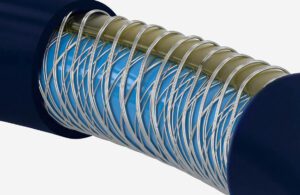
The Cerenovus Emboguard balloon guide catheter’s braiding offers flexibility and stiffness for navigating to clots for removal. [Photo courtesy of Johnson & Johnson MedTech]
In terms of simplicity, aspiration catheters are relatively straightforward devices that use vacuum suction to grab and remove clots blocking blood flow to the brain.
“What we’re seeing is a preference in some cases just to go with direct aspiration rather than using a stent retriever,” Gilvarry said. “Like a lot of cases in medical devices, ease of use drives a lot of the trends over time. With aspiration, the bigger the catheter, the more effective it is. You can do the math on that quite easily.”
Some procedure set-ups combine aspiration and stent retrieval, while others use balloons to reduce blood pressure and flow behind the clot to aid in removal. Those balloon catheters have historically been viewed as stiffer and more difficult to navigate past the aortic arch, Gilvarry said, but Cerenovus designed its braided Emboguard balloon guide catheter with an eccentric lumen for better trackability.
“The technology we used there also includes variable durometer polymers that create the jacket on the outside of it, the pitch of the braid,” Gilvarry said. “But all the time you’re trying to push the limitations of what you can do with these technologies. Essentially what you’re trying to do is create the smallest possible puncture site [while] trying to maximize the inter lumen to get these aspiration catheters through it for the biggest interface you can have with a clot.”
Because thrombectomy patients are often awake during the procedure, Cerenovus also designed the balloon catheter to have some give in case the patient moves their head.
“A doctor said to me one time that there is no procedure in medicine as rewarding as treating a stroke patient,” Gilvarry said. “Because when you unblock the vessel, patients can have an immediate recovery. It’s as dramatic as being able to suddenly remember or recognize what’s going on. A doctor will often ask them to move their arms or lift their leg, and they can do that even in the angio suite.”
Developing and testing thrombectomy devices

A blod clot captured by the Embotrap stent retriever [Photo courtesy of Johnson & Johnson MedTech]
“We embarked on this journey back in 2010 when there was still very little known about what type of clots you’re dealing with,” he said. “But what was established is if you can restore blood flow, then you get a treatment effect. For us as design engineers, that’s a very nice definition of what you’re trying to achieve in a device. You’re trying to remove the blood clot, you’re trying to do it quickly because time is brain, and you’re trying to do it in one pass.”
The team set out to understand the different physical properties of clots in order to recreate them in vascular models and test device prototypes for reaching and removing blockages. They considered synthetic materials to simulate the clots, but stuck with actual blood.
“The polymer structure of synthetic materials or even materials like cellulose is quite different to the microstructure of the clot, and therefore the behavior is different,” Gilvarry said.
They focused their prototype designs on simplicity and speed plus effectiveness, using hundreds of models to simulate the tortuous anatomy of the brain and a broad representation of different patients.
“We did a lot of experiments to try and fail quickly,” he said. “That was the mantra: build prototypes quickly, but learn from it each time you build and then iterate to change whatever it is about the device design that you’re trying to change.”
The models recreated the anatomies of the aortic arch anatomy, the cervical carotid and the brain vessels where the devices encounter the clots. The team targeted different variations that could be difficult for catheters to navigate.
“Sometimes you see this pretty nasty S bend in the carotid artery, like a kink almost,” Gilvarry said. “You have to try and navigate past that, get around the carotid siphon where the carotid enters the skull base and starts going into the brain. A variance there can be challenging to cross with catheters.”
The team also used finite element analysis to guide them on the appropriate stiffness profile.
“You use that to select your geometry of materials or your braiding density in order to give you the kind of stiffness that you’re trying to aim for. You still have to test it in your models and different anatomies. But it’s kind of a shortcut to getting to a design a bit quicker than you might by just pure trial and error.”
Above all else, Gilvarry emphasized the importance for medtech designers to spend time understanding the problem they’re trying to solve and recreating the problem with complex models.
“It’s pushing the performance and the innovation envelope all the time, continuously iterating, innovating and setting the bar high enough. Because if you don’t, if your model is too simple and it doesn’t replicate everything, there’s a lot of nuance to these challenges,” he said. “You have to replicate all of those nuances. … It takes patience to get to that point, but it certainly has worked for us.”
No comments:
Post a Comment