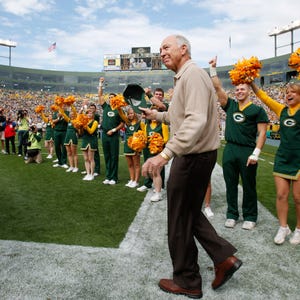The unasked question. 'What proof have your doctors given you that these stem cells caused your improvement?' Without that answer, this is all just placebo and magic. If the stem cell company or researchers you are following don't mention tracking of stem cells then they have NO fucking clue if they survive or not.
What should be used to check the health of stem cells:
Tracking of Administered Progenitor Cells in Brain Injury and Stroke by Magnetic Resonance Imaging
The problems that can occur:
Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient.
He went abroad for stem cell treatment. Now he’s a cautionary tale. Stroke patient Jim Gass
And the credulous article on Bart Starr:
Wearing a sporty black Green Bay Packers polo shirt, Bart Starr got off of a private jet here last week and walked into the California sunshine.
This
was quite a feat. Starr, 82, had suffered two strokes and a heart
attack in 2014, followed by a bronchial infection that nearly killed him
in 2015 and then a broken hip that put him back in a wheelchair
in December.Just a few weeks ago, the legendary former Packers quarterback even underwent surgery to remove stones from his bladder.
Yet here he was again, on his feet and smiling after flying in for another round of experimental stem cell treatments 20 miles south of here, in Tijuana, Mexico.
“Three times,” Starr interjected when asked by USA TODAY Sports about his visits here since June 2015.
“We’re really looking for big things to happen with this visit because this is probably the healthiest Bart’s been in two years,” said Cherry Starr, Bart’s wife.
Cherry Starr believes her husband has bounced back from his health setbacks with help from these treatments, the same kind of treatments received by two other famous stroke victims: hockey great Gordie Howe and former NFL MVP quarterback John Brodie.
The treatments involve a combination of stem cells derived from donated bone marrow and fetal brain tissue. They are manufactured by a company in San Diego, Stemedica, but they are not approved for use in the USA.
To gain such approval, they first would have to pass years of expensive tests on their safety and effectiveness.
But Brodie, 81, Starr and Howe, who died in June, didn’t believe they had time to wait. They also believed they had little to lose by going to Mexico, where the company has said it’s more cost-effective to have its products tested than in the USA. Stemedica ships the cells to a sleek, modern clinic in an old, run-down part of Tijuana, not far from various dental clinics and a taco restaurant.
“When I first went down there, I didn’t really know what to expect,” Cherry Starr said Thursday after returning home to Alabama. “I thought,`Oh my gosh, what are we getting into?’ when we were driving through town. But it’s a lovely, lovely facility.”
Clinica Santa Clarita in Tijuana has had about 250 patients take part in its clinical trial over the past two years. Patients are often charged for these experimental treatments, which cost $30,000. Bart Starr also paid for his treatment, Cherry said.
His treatment involved an injection of fetal-derived neural cells into his spine last week, followed by an injection of bone marrow-derived mesenchymal cells into his arm the next day.
Afterward, Cherry Starr said “everything went absolutely great” but that it was too early to notice any possible effects. Bart Starr, a Pro Football Hall of Famer, can walk slowly and usually with help at his sides. His speech also is limited, but it’s still a big improvement from being wheelchair-bound and barely able to feed himself after his stroke two years ago.
“Stem cells, wow!” said Brodie, who greeted Starr here Tuesday and is a former rival of Starr’s as quarterback of the San Francisco 49ers.
American stem cell experts stress caution about such treatments, either in the USA or especially in other countries where safety and effectiveness standards might not be as strict. For example, it’s not known how much Starr, Brodie or Howe improved on their own through natural healing or physical therapy.
Larry Goldstein, a stem cell expert and professor at the University of California San Diego, said it takes at least five to 10 years to test whether such treatments really work. In the meantime, few stem cell treatments are approved for use in the USA, though many American clinics have been offering legally and scientifically questionable stem cell treatments derived from a patient’s own fat or bone marrow.
Stemedica’s stem cells are different than these because they are derived from donors and are replicated in a lab. They are considered unapproved biological drugs in the USA.
“There is definitely a wild west of clinics in the U.S. and outside the U.S. offering unproven treatments of stem cells,” Goldstein told USA TODAY Sports in June.
Stemedica is working toward approval by the U.S. Food and Drug Administration, including with a clinical trial in Toledo for traumatic brain injury patients. It involves bone marrow-derived cells and is part of the Gordie Howe Initiative, named after perhaps the most famous stem cell patient in America. Before his death at age 88, his family credited the Tijuana treatments with boosting the last year and a half of his life after his stroke.
On Aug. 29, a subsidiary of Stemedica announced that data from a separate, pre-clinical study showed that its bone marrow-derived stem cells improved heart function after heart attacks.
“We’ve always known that our stem cells exhibit unique qualities and characteristics, and we’re very proud of what people call the `anecdotal evidence’ coming from patient testimonials,” said Dave McGuigan, Stemedica’s vice president for marketing and business development. “But now that we can give the academic and scientific community statistical evidence to go alongside the anecdotal meaningful evidence, that can only help add to the Stemedica story.”
Follow sports reporter Brent Schrotenboer on Twitter @Schrotenboer. E-mail: bschrotenb@usatoday.com