Good word salad, BUT NOTHING EXACT FOR UPPER LIMB RECOVERY!
Useless.
Did your doctor do anything with this in the past 6 years? NO? Then you don't have a functioning stroke hospital.
Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery
Front. Hum. Neurosci., 13 September 2016
Sec.Motor Neuroscience
https://doi.org/10.3389/fnhum.2016.00442
- 1Physical and Rehabilitation Medicine, Brugmann University Hospital, Brussels, Belgium
- 2Systems and Cognitive Neuroscience, Institute of Neuroscience, Université Catholique de Louvain, Brussels, Belgium
- 3Faculty of Medicine and Pharmacy, Faculty of Physical Education and Physiotherapy, Vrije Universiteit Brussel, Brussels, Belgium
- 4Physical and Rehabilitation Medicine, Centre Hospitalier de l'Ardenne, Libramont, Belgium
- 5Movement Control and Neuroplasticity Research Group, Motor Control Laboratory, Department of Kinesiology, Katholieke Universiteit Leuven, Leuven, Belgium
- 6Physical Medicine and Rehabilitation, Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Brussels, Belgium
Stroke is one of the leading causes for disability worldwide. Motor
function deficits due to stroke affect the patients' mobility, their
limitation in daily life activities, their participation in society and
their odds of returning to professional activities. All of these factors
contribute to a low overall quality of life. Rehabilitation training is
the most effective way to reduce motor impairments in stroke patients.
This multiple systematic review focuses both on standard treatment
methods and on innovating rehabilitation techniques used to promote
upper extremity motor function in stroke patients. A total number of
5712 publications on stroke rehabilitation was systematically reviewed
for relevance and quality with regards to upper extremity motor outcome.
This procedure yielded 270 publications corresponding to the inclusion
criteria of the systematic review. Recent technology-based interventions
in stroke rehabilitation including non-invasive brain stimulation,
robot-assisted training, and virtual reality immersion are addressed.
Finally, a decisional tree based on evidence from the literature and
characteristics of stroke patients is proposed. At present, the stroke
rehabilitation field faces the challenge to tailor evidence-based
treatment strategies(NOT PROTOCOLS!) to the needs of the individual stroke patient.
Interventions can be combined in order to achieve the maximal motor
function recovery for each patient. Though the efficacy of some
interventions may be under debate, motor skill learning, and some new
technological approaches give promising outcome prognosis in stroke
motor rehabilitation.(There is nothing promising if you don't have protocols.)
Introduction
The World Health Organization (WHO) estimates that stroke events in EU countries are likely to increase by 30% between 2000 and 2025 (Truelsen et al., 2006). The most common deficit after stroke is hemiparesis of the contralateral upper limb, with more than 80% of stroke patients experiencing this condition acutely and more than 40% chronically (Cramer et al., 1997). Common manifestations of upper extremity motor impairment include muscle weakness or contracture, changes in muscle tone, joint laxity, and impaired motor control. These impairments induce disabilities in common activities such as reaching, picking up objects, and holding onto objects (for a review on precision grip deficits, see Bleyenheuft and Gordon, 2014).
Motor paresis of the upper extremity may be associated with other neurological manifestations that affect the recovery of motor function and thus require focused therapeutic intervention. Deficits in somatic sensations (body senses such as touch, temperature, pain, and proprioception) after stroke are common with prevalence rates variously reported to be 11–85% (Carey et al., 1993; Yekutiel, 2000; Hunter, 2002). Functionally, the motor problems resulting from sensory deficits after stroke can be summarized as (1) impaired detection of sensory information, (2) disturbed motor tasks performance requiring somatosensory information, and (3) diminished upper extremity rehabilitation outcomes (Hunter, 2002). Sensation is essential for safety even if there is adequate motor recovery (Yekutiel, 2000). Also, up to 50% of patients experience pain of the upper extremity during the first year after stroke, especially shoulder pain and complex regional pain syndrome-type I (CRPS-type I), which may impede adequate early rehabilitation (Jönsson et al., 2006; Kocabas et al., 2007; Sackley et al., 2008; Lundström et al., 2009). Furthermore, joint subluxation and muscle contractures can lead to nociceptive musculoskeletal pain (de Oliveira et al., 2012). Among other complications of stroke the neglect syndrome (Ringman et al., 2004) and spasticity (Sommerfeld et al., 2004; Welmer et al., 2010) affect motor and functional outcomes.
The neurological recovery after stroke displays a nonlinear, logarithmic pattern (Figure 1; Kwakkel et al., 2006; Langhorne et al., 2011). The greater part of recovery is reported to take place in the first 3 months following stroke (Wade et al., 1983). However, there is evidence that recovery is not limited to this time period; hand and upper extremity recovery has been reported many years after stroke (Carey et al., 1993; Yekutiel and Guttman, 1993). Improvement probably occurs through a complex combination of spontaneous and learning-dependent processes including: restitution, substitution, and compensation (Kwakkel et al., 2004; Langhorne et al., 2011). Until the third month after stroke onset, a variable spontaneous neurological recovery can be considered a confounder of rehabilitation intervention (Kwakkel et al., 2006). In the past, the observation of spontaneous recovery after stroke has misled some authors to believe that recovery of upper extremity function is intrinsic and that little can be done by therapists to influence it (Wade et al., 1983; Heller et al., 1987). Progresses in functional outcome appearing after 3 months seem largely dependent on learning adaptation strategies (Kwakkel et al., 2004). Evidence suggests that neurological repair through brain reorganization supporting true recovery or, alternatively through compensation, may also take place in the subacute and chronic phase after stroke (Krakauer, 2006).
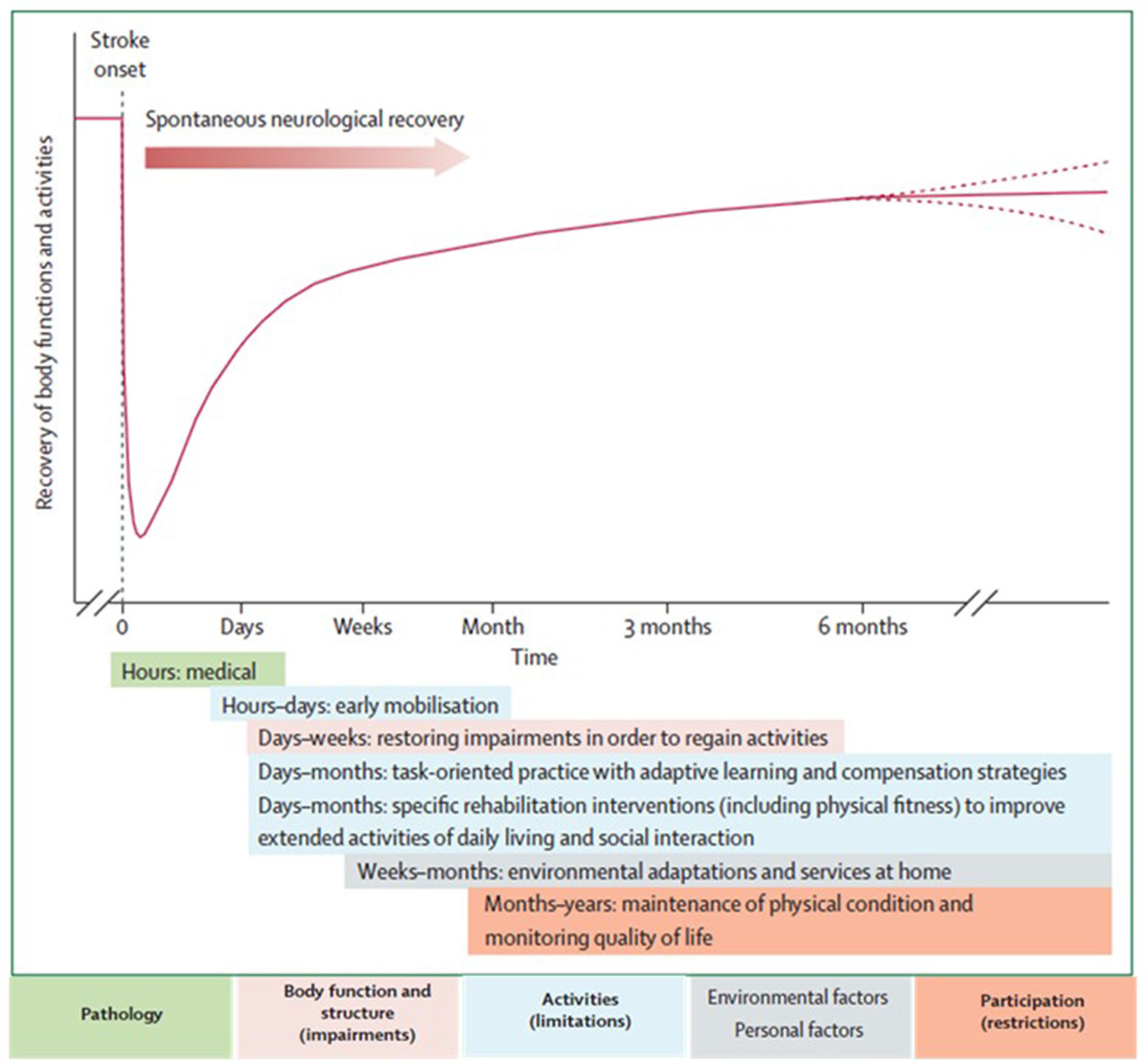
Figure 1. Hypothetical pattern of recovery after stroke with timing of intervention strategies. The neurological recovery after stroke displays a nonlinear, logarithmic pattern. The greater part of recovery is reported to take place in the first three months following stroke. Rehabilitation interventions targeting at improving a stroke patients' performance should be implemented according to the phase of neurological recovery. Reprinted from Langhorne et al. (2011), Copyright [2011] by Elsevier. Reprinted with permission.
Functional imaging of stroke recovery corroborates this temporal pattern of activation shifts. Shortly after stroke, an initial contralesional shift of activation toward the “unaffected” hemisphere is observed, followed by the activation of learning-related brain structures (including the cerebellum, basal ganglia, and frontal cortices) (Hikosaka et al., 1998; Lehéricy et al., 2005). Finally, two activation patterns are described depending on the degree of recovery (related to the amount of remaining fibers in the impaired corticospinal tract), either a perilesional (refocusing), or a distributed recruitment pattern (Feydy et al., 2002; Ween, 2008). Rehme et al. (2012) confirmed this last assumption and concluded that a good functional outcome relies on the recruitment of the original functional network rather than on contralesional activity. The meta-analysis by Richards et al. (2008) concluded that brain activations increase within the lesioned hemisphere after an upper extremity rehabilitation program. Brain plasticity including reorganization and compensation processes is the base for neurological recovery, as described above, however the exact pathophysiological mechanisms underlying rehabilitation's efficacy remain unclear (Eliassen et al., 2008).
Stroke recovery is heterogeneous in terms of functional outcome. Patients with mild to moderate upper extremity paresis in acute phase have a good prognosis for functional recovery, as 71% of these patients achieve at least some dexterity at 6 months after stroke (Nijland et al., 2010). The prognosis in severely affected patients is poor with about 60% failing to achieve some dexterity at 6 months after stroke (Kwakkel et al., 2003; van Kuijk et al., 2009). Finally, only 5% of patients who initially experienced complete paralysis achieve functional use of their arm. Upper extremity impairments chronically affect the functional independence and satisfaction in 50–70% of all stroke patients. (Bonita and Beaglehole, 1988).
Algorithms have been developed to predict motor function recovery after stroke (Stinear et al., 2007). Predictor variables include age, sex, lesion site, initial motor impairment, motor-evoked potentials, and somatosensory-evoked potentials. Initial measures of upper extremity impairment and function were found to be the most significant predictors of upper extremity recovery (Coupar et al., 2012). Findings so far suggest that the first assessments should be quick and simple, such as bedside tests of motor impairment, with progression to more complex tests if uncertainty remains (Figure 2). Later tests can include neurophysiological assessments and neuroimagery of the motor system integrity.
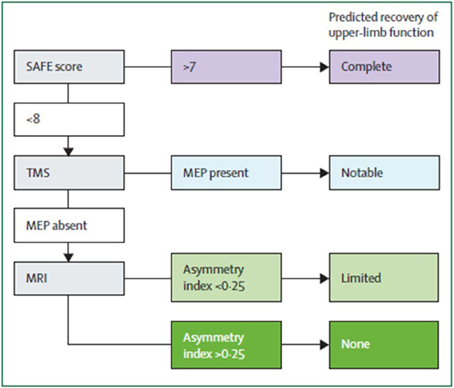
Figure 2. Suggested sequence of tests to predict the recovery of motor function in patients with subacute stroke (weeks after stroke). Although this particular algorithm requires validation, it illustrates a potentially efficient progression from simple to more complex predictive measures. SAFE, sum of muscle force on shoulder abduction and finger extension according to Medical Research Council muscle grades at 72 h after stroke; TMS, transcranial magnetic stimulation; MEP, motor evoked potentials in the affected upper limb; Asymmetry index, asymmetry index of fractional anisotropy in the posterior limbs of the internal capsules measured with diffusion-weighted MRI. From Stinear et al. (2014).
Interdisciplinary complex rehabilitation interventions represent the mainstay of post-stroke care (Langhorne and Legg, 2003; Langhorne et al., 2011). Stroke rehabilitation aims at providing all possible means to recover lost function and to increase the autonomy of stroke patients taking into account the remaining impairments and disabilities. Carr and Shepherd (2011) suggested that poor upper extremity recovery may be due to the direct impact of the stroke itself as well as to insufficient, inadequate or inappropriate therapeutic interventions. Little information is available, however, to describe what best represents “optimum treatment” (Ballinger et al., 1999). From a theoretical point of view, a stroke rehabilitation program for upper extremity motor impairment should include global motor rehabilitation, electrical brain stimulation, hemispheric subspecialization in motor activities, and multisensory interaction (Johansson, 2011). A recent Cochrane review focussing on the recovery of function and mobility in stroke patients reported the potential benefit of rehabilitation therapy on motor impairments and disabilities, compared with no treatment, in function of the time since stroke (Pollock et al., 2014). While these type of systematic reviews and meta-analyses are very powerful, they only take into account rehabilitation techniques that already have been reported in other systematic reviews and may thus ignore rehabilitation approaches that pertain to the routine clinical setting. Furthermore, in most systematic reviews only randomized controlled trials are reported.
The purpose of the present manuscript was to undertake a systematic review for each of the neurorehabilitation techniques that may be useful in promoting upper extremity motor recovery. The search terms and inclusion criteria of reported trials have been chosen as large as possible in order to detect pertinent information on rehabilitation methods that are currently used in clinical practice, but are uncommonly discussed in systematic reviews (examples: music therapy, motor skill learning, isokinetic muscle strengthening, paired associative stimulation, theta burst stimulation). In some cases, routine clinical treatments that have not been investigated in a randomized controlled way, are still included in the present systematic review if the trial demonstrated sufficient quality evidence. The scientific evidence of each stroke rehabilitation intervention is discussed and presented with a practical recommendation for clinicians working in the field of neurorehabilitation. A decisional tree according to the patient's characteristics is proposed based on scientific evidence available for the different interventions.
More at link.
 Samar M. Hatem
Samar M. Hatem Geoffroy Saussez2,
Geoffroy Saussez2,  Yannick Bleyenheuft
Yannick Bleyenheuft
No comments:
Post a Comment