159 references which I'm sure your competent? doctor is quite familiar with since stroke is a hypoxic condition. What will your doctor do with this information to save the penumbra?
The Application of Drugs and Nano-Therapies Targeting Immune Cells in Hypoxic Inflammation
Back to Journals » International Journal of Nanomedicine » Volume 19
Authors Luo J, Wang H, Chen J, Wei X, Feng J, Zhang Y, Zhou Y
Received 9 January 2024
Accepted for publication 29 March 2024
Published 9 April 2024 Volume 2024:19 Pages 3441—3459
DOI https://doi.org/10.2147/IJN.S456533
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Mian Wang
Jiaxin Luo,1,2,* Hanchi Wang,1,2,* Jingxia Chen,1,2 Xuyan Wei,1,2 Jian Feng,1,2 Yidi Zhang,1,2 Yanmin Zhou1,2
1Jilin
Provincial Key Laboratory of Tooth Development and Bone Remodeling,
Hospital of Stomatology, Jilin University, Changchun, 130021, People’s
Republic of China; 2Department of Oral Implantology, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China
*These authors contributed equally to this work
Correspondence:
Yidi Zhang; Yanmin Zhou, Department of Oral Implantology, Hospital of
Stomatology, Jilin University, Changchun, 130021, People’s Republic of
China, Email zhangyidi@jlu.edu.cn; zhouym@jlu.edu.cn
Abstract:
Immune cells are pivotal in the dynamic interplay between hypoxia and
inflammation. During hypoxic conditions, HIF-1α, a crucial transcription
factor, facilitates the adaptation of immune cells to the hypoxic
micro-environment. This adaptation includes regulating immune cell
metabolism, significantly impacting inflammation development. Strategies
for anti-inflammatory and hypoxic relief have been proposed, aiming to
disrupt the hypoxia-inflammation nexus. Research extensively focuses on
anti-inflammatory agents and materials that target immune cells. These
primarily mitigate hypoxic inflammation by encouraging M2-macrophage
polarization, restraining neutrophil proliferation and infiltration, and
maintaining Treg/TH17 balance. Additionally, oxygen-releasing
nano-materials play a significant role. By alleviating hypoxia and
clearing reactive oxygen species (ROS), these nano-materials indirectly
influence immune cell functions. This paper delves into the response of
immune cells under hypoxic conditions and the resultant effects on
inflammation. It provides a comprehensive overview of various therapies
targeting specific immune cells for anti-inflammatory purposes and
explores nano-materials that either carry or generate oxygen to
alleviate anoxic micro-environments.
Keywords: hypoxia, inflammation, immunometabolism, immunotherapy, nanotherapy
Introduction
The recognition that hypoxia can precipitate inflammation is now widely acknowledged. A key feature of acute respiratory distress syndrome (ARDS) is hypoxemia. In ARDS patients, a notable decrease in monocytes accompanies this hypoxemia. Thus, by establishing a mouse model of acute lung injury, Ananda found that impaired monopoiesis led to reduced accumulation of monocyte-derived macrophages and enhanced neutrophil-mediated inflammation in the lungs.1 Moreover, cycling hypoxia may occur in the tumor area due to intermittent flow of red blood cells into tumor vessels. Scholars have demonstrated that cycling hypoxia can affect the polarization of tumor-associated macrophages (TAMs) and activate endothelial cell activation through nuclear factor-kappa B (NF-kB) pathway, thus promoting tumor inflammation.2 Furthermore, hypoxia can promote anaerobic glycolysis, resulting in increased production and release of lactic acid. Prior studies have indicated that lactate escalates the differentiation of T helper 1 cells and the generation of interferon-γ (IFN γ). Additionally, the augmentation of lactic acid encourages M2-like polarization and vascular endothelial growth factor (VEGF) expression in tumor-associated macrophages (TAMs). This is partially mediated by the activation of hypoxia-inducible factor 1α (HIF1α).3,4 Under normoxic conditions, HIF-1α is rendered inactive through hydroxylation by prolyl hydroxylases (PHDs) and factor-inhibiting HIF (FIH). In hypoxic states, these hydroxylases are inactivated due to the scarcity of available non-mitochondrial oxygen, leading to the stabilization and transcription of HIF.5 HIF expression occurs in innate immune cells, including macrophages, neutrophils, and TH17 cells. As a principal transcriptional regulator of immune cell function, HIF aids these cells in adapting to hypoxic environments and enhances the expression of inflammatory genes.6 The relationship between hypoxia and inflammation is synergistic, not merely cause-and-effect. Hypoxia in inflammatory conditions arises from the recruitment of immune cells, the increased metabolic demands of various cells, and the depletion of metabolic substrates due to trauma and compression.7,8 In inflamed tissues, infiltrating immune cells alter the local tissue micro-environment by depleting molecular oxygen. Studies have revealed that activated neutrophils can promote HIF-1α transcription by consuming local oxygen, particularly when epithelial cells detect proximate hypoxia.9 Moreover, the proliferation of intracellular pathogens can exacerbate oxygen deprivation in infected tissues.7
To address the interplay between hypoxia and inflammation, strategies focusing on anti-inflammatory actions and hypoxic mitigation have been developed. Bio-active drugs and materials that target immune cells primarily curb hypoxic inflammation by influencing the functionalities of macrophages, neutrophils, T cells, and other immune constituents.10,11 Additionally, recent advancements have brought forth various nano-therapies designed to supply or generate oxygen at inflammatory sites. These include hemoglobin-based oxygen carriers, perfluorocarbon-based oxygen carriers, catalase-mediated oxygen generation, nano-enzyme-mediated oxygen generation, and metal peroxide decomposition.12 However, comprehensive reviews detailing how hypoxia influences inflammation’s onset and progression, and the application of drugs or bio-active nano-materials for treating inflammation under hypoxic conditions, particularly from an immune cell perspective, are scarce. Based on immune cells, this paper examines the alterations in immune cells under hypoxic conditions and their implications for inflammation. It methodically reviews different therapies that target various immune cells to exert anti-inflammatory effects. In addition to bio-active drugs and materials targeting immune cells, the paper also thoroughly evaluates nano-materials capable of transporting or generating oxygen to alleviate anoxic micro-environments. This exploration aims to uncover more bio-active materials for the prevention and treatment of hypoxic inflammation, with a focus on underlying mechanisms.
The Mechanism of Inflammation Under Hypoxia
The host immune response is typically categorized into innate and adaptive immune responses. The innate response is rapid and non-specific against pathogens, whereas the adaptive response, characterized by antigen receptor gene rearrangement, develops slowly but with specificity, leading to classical immune memory.13
Hypoxia and Innate Immunity
This section focuses on two primary innate immune cells: macrophages and neutrophils. Macrophages exhibit diverse activation states influenced by their local environment, broadly categorized into classically activated M1 macrophages and alternatively activated M2 phenotypes. While M1 macrophages are generally pro-inflammatory, M2 macrophages are regarded as “immunomodulatory”, playing roles in wound healing and anti-inflammation.14 Neutrophils, significant in inflammation, exacerbate the inflammatory state by aiding macrophage recruitment and interacting with antigen-presenting cells.15 In addition, other innate immune cells, like dendritic cells, natural killer cells, and mast cells, also variably impact inflammation under hypoxic conditions.
Hypoxia and Macrophage
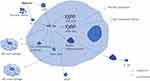
Hypoxia triggers increased hypoxia signaling, lactic acid buildup, and metabolic acidosis (Figure 1). Studies have shown that hypoxia elevates HIF-α secretion.16,17 Hypoxia initially inhibits PHD activity, reducing PHD’s suppressive effect on HIF-α. Additionally, IL-1, induced by cancer necrotic debris under severe and prolonged hypoxia, augments HIF-α synthesis.18 Elevated HIF-α is crucial for macrophage differentiation and inflammatory chemokine release. HIF-1α stabilizes the long noncoding RNA (HISLA), a myeloid-specific lnc-RNA, influencing miR-210 and miR-383 levels in lesion-associated macrophages, affecting their aerobic glycolysis and anti-apoptotic capabilities.19,20 Furthermore, the mTOR/HIF-1α/glycolysis pathway activation is a novel mechanism for NLRP3 inflammasome activation in macrophages.21 The NLRP3 inflammasome, a multi-protein complex, facilitates pro-inflammatory cytokine IL-1β secretion in a caspase-1-dependent manner, regulating inflammation.22 Along with the NLRP3 inflammasome, HIF-1α promotes the polarization of M1 macrophages,23 which leads to the release of pro-inflammatory cytokines such as TNF-α, IL-1, nitric oxide, and other chemokines. The increase in IFN-γ levels, mediated by HIF-1α, significantly influences macrophage cytokine production, antigen-presenting activity, and phagocytic function in hypoxic conditions.24
In conditions of mild hypoxia (4–8% Oxygen), the metabolic alterations mediated by HIF-1α-PDK1 significantly contribute to macrophage migration and activation, critical processes in inflammation.25 During the inflammatory activation of macrophages, both HIF-1α and glycolytic pathway metabolites can intensify inflammation. Hypoxia and inflammation collectively enhance anaerobic and aerobic glycolysis, leading to increased lactic acid release and metabolic acidosis.3 Lactic acid plays a crucial role in signaling, notably by inducing vascular endothelial growth factor expression and M2 macrophage polarization.26 In anaerobic tumor environments, lactic acid activates mTORC1, which in turn inhibits TFEB-mediated expression of the macrophage-specific vacuolar ATPase subunit ATP6V0d2, targeting HIF-2α. The absence of ATP6V0d2 results in increased macrophage polarization, elevated VEGF production, and enhanced HIF-2α stability.27 HIF-2α elevates Arg-1 expression in macrophages, promoting M2 macrophage polarization.28 M2 macrophages aid in resolving inflammation, tissue repair, and cell proliferation by releasing anti-inflammatory cytokines like IL-10 and clearing necrotic cell debris.29
Hypoxia and Neutrophil
Neutrophils are the most important component of innate immunity which play an important role in inflammation. When inflammation occurs, neutrophils migrate across the vascular barrier along the concentration of cytokines to the site of inflammation. In hypoxia, HIF is activated which is controlled by the oxygen sensors of neutrophils such as prolyl and asparaginyl hydroxylase enzymes.30 In colitis-associated colon cancer model mice, over-expression of HIF-2α in the intestinal epithelium promotes the recruitment of neutrophiles to the colon and regulates the infiltration to tumor-associated neutrophils to regulate the colon tumor micro-environment.31 As the neutrophils arrive the area of inflammation, the main functions of neutrophils are phagocytosis, production of reactive oxygen species (ROS), release of inflammatory proteins, and apoptosis.32 Neutrophils maintain polarized mitochondria, produce ROS and regulate HIF-1α stability by using the glycerol 3-phosphate pathway as a way of directly regulating mitochondrial function through glycolysis.33 Enhanced HIF1-α expression up-regulates critical glycolytic enzymes, including G3PDH and triosephosphate isomerase-1, thus increasing energy production and cell viability. Additionally, HIF-1α serves as an upstream regulator of NF-κβ, augmenting pro-inflammatory cytokine production and thereby extending inflammation.34,35 Conversely, hypoxia may induce neutrophil degranulation, potentially due to an initial HIF-independent phase involving PI3Kγ signaling pathway activation, or a subsequent HIF-dependent gene transcription response.30 This heightened degranulation in hypoxic conditions aids in pathogen clearance and enhances interactions with other immune cell types in neutrophils.36 However, research indicates that increased neutrophil degranulation under hypoxic conditions may lead to the release of harmful substances, like active nitrogen and toxic proteases, causing local tissue toxicity.37,38 Moreover, a dual host defense mechanism, neutrophil extracellular traps (NETs), is released via autophagy-associated signaling pathways, controlled by HIF-1α through the neutrophil stress-response protein REDD1.39 NETs combat bacteria but can also damage tissue and blood vessels during inflammation.40 Dysregulated NETs formation during pathological stimulation can result in sepsis and systemic inflammatory response syndrome.41 Furthermore, hypoxia may delay apoptosis and regulate neutrophil retention at inflammation sites through HIF-1α stabilization and NF-κ B activation.35
Hypoxia and Other Innate Immune Cells
Dendritic cells (DCs) form a vital link between innate and adaptive immunity, with the key function of recognizing pathogens and presenting antigenic peptides to T cells.42 Initially, human-derived monocytes differentiate into DCs and migrate to peripheral tissues, where immature DCs assess the environment and gather antigenic materials.43 However, some studies suggest that hypoxia down-regulates the uptake of antigens and phagocytosis in DCs, in a process seemingly independent of HIF-1α.42 Following antigen phagocytosis, DCs travel to draining lymph nodes via afferent lymphatic vessels to present the antigens to T cells.43 Research indicates that hypoxia inhibits the migration of human monocyte-derived DCs in vitro by reducing MMP-9 expression and increasing protease inhibitor TIMP1 levels.44,45 Conversely, other studies have shown that hypoxia enhances the migratory capability of DCs via the HIF-1α and PI3K/Akt pathways.43 DCs present antigens to T cells by expressing surface costimulatory molecules, prompting T cells to release specific cytokines.46 Yet, the impact of hypoxia on the expression of co-stimulatory molecules (eg, CD80, CD83) and the release of pro-inflammatory cytokines remains a topic of debate. Reports have shown that hypoxia can lead to a decrease,47,48 an increase,49,50 or no change51 in the expression of surface co-stimulatory molecules. Additionally, hypoxia has been reported to have both stimulatory42,46,49,51 and inhibitory42,48 effects on cytokine release (eg, interleukin (IL)-6, IL-10, IL-12, and TNF-α). The variability in these effects can be attributed to: (1) different environments in which DCs are induced and matured in vitro; (2) variations in the types of co-stimulatory molecules on human and animal DCs.48 (3) The interaction between the TLR signaling pathway and HIF-1α, leading to varied immune responses in different diseases.46 Consequently, further in vivo research is essential to better understand the delicate balance between necessary immune induction to combat diseases and the excessive activation of immune cells that can harm the host.
Natural killer (NK) cells, a type of cytotoxic innate lymphoid cells, induce apoptosis in target cells by up-regulating death-inducing ligands such as Fas-L and TRAIL and producing various pro-inflammatory cytokines and chemokines.52 Hypoxia sustains NK cell glycolysis by enhancing HIF-1α expression and inhibiting NK cell apoptosis via the HIF-1α/NAD axis.53,54 Moreover, hypoxia alone can diminish NK cell cytotoxicity by reducing the phosphorylation levels of ERK and STAT3 in a SHP-1-dependent manner,52 but it does not increase adhesion molecules or NK cell adhesion to human endothelial cells.55 Contrarily, Maurus demonstrated that the combination of TNF-α, a pro-inflammatory cytokine, with hypoxia augments the expression of adhesion molecules like ICAM-1 and enhances NK cell adhesion.55 Anti-inflammatory treatment strategies have been shown to mitigate hypoxia-induced damage. Mast cells, pivotal in coordinating inflammatory processes, produce and release abundant inflammatory cytokines.56 Hypoxia preserves the degranulation ability of mast cells and boosts their proliferation and secretion of pro-inflammatory cytokines, such as TNF-α and IL-6.57 Additionally, mast cells express various adhesion molecules, including integrin receptors, crucial for their localization and penetration into inflammatory tissue sites.56 Hypoxia down-regulates mast cell adhesion to hyaluronic acid by decreasing hyaluronic acid receptor affinity,58 but up-regulates mast cell adhesion to fibronectin via the PI3K/AKT signaling pathway.56 Both hyaluronic acid and fibronectin are key components of the extracellular matrix.58 These contrasting findings indicate that hypoxia significantly influences mast cell interactions with the extracellular matrix, potentially playing a role in mast cell accumulation at disease sites.
Hypoxia in Adaptive Immunity
T cells and B cells constitute the primary components of adaptive immunity. T cells differentiate into CD4+ and CD8+ subsets, marked by specific surface proteins. CD8T cells exhibit cytotoxicity, capable of directly eliminating damaged and cancerous cells. In contrast, CD4+ helper T cells indirectly induce cellular damage. Upon activation, CD4 T cells diverge into subpopulations influenced by local antigens and cytokines. These subpopulations of helper T cells are instrumental in eradicating specific microbial pathogens and stimulating pro-inflammatory responses in other immune cells. T follicular helper (Tfh) cells enhance the survival and proliferation of germinal center B cells, while regulatory T cells (Tregs) are crucial in maintaining immune homeostasis and self-tolerance, mitigating inflammation, and preventing autoimmune diseases.59,60 Activated T cells propel B cell development, whereas soluble antigens stimulate B cells through B cell receptors, leading to antibody responses and the generation of memory B cells alongside CD8 memory T cells. These memory cells are primed for rapid proliferation upon subsequent pathogen encounters.59
Hypoxia and T Cells
In hypoxic conditions, T cells are activated and drawn to inflammation sites. HIF-1α associates with a transcriptionally active hypoxia-response element (HRE) in the PD-L1 proximal promoter, inhibiting PD-L1 activation in hypoxia. This enhances T cell activation while downregulating IL-6 and IL-10.61 Hypoxia impacts CD8 T cell glycolytic metabolism, their ability to eliminate target cells, and infiltration, all in an HIF-1α-dependent manner.62 HIF-1α is crucial for CD8 T cells’ effector state. Hypoxia fosters CD8 T cell differentiation via the IL-4-HIF-1α-IL-13 axis, enhancing CD8 T cell-dependent airway hyperreactivity and inflammation, potentially contributing to steroid-refractory asthma development.63 Additionally, IL-2, a vital cytokine for preventing chronic inflammation, upholds high levels of glucose metabolism and glycolysis in CD8 T cells by maintaining mTORC1 activity and HIF1α protein expression.64,65 HIF also regulates CD4 T cell populations by promoting CD154 expression on Tfh cells, vital for stimulating CD40 on germinal center B (GCB) cells. This affects the quantity of switch cytokines secreted by differentiated helper cells and alters the metabolic programming of activated T cells. Hypoxia diminishes cytokine secretion upon re-stimulation of differentiated CD4 T cells and modifies their metabolic programming. Conversely, HIF enhances the capacity of activated CD4 T cells to produce switch cytokines.66
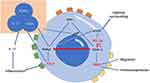
Regulatory T cells (Tregs), a specialized subpopulation of CD4 T cells, are essential in suppressing T cell activation, maintaining peripheral immune tolerance, and curbing inflammatory responses.67 The impact of hypoxia on Tregs is multifaceted and somewhat contradictory. Clambey demonstrated that Treg-intrinsic HIF-1α significantly restrains inflammation. He observed that hypoxia in mucosal inflammation stimulates Foxp3 transcription via HIF-1α, thereby enhancing the abundance and functionality of Tregs.68 Foxp3 counters the activity of the retinoid-related orphan receptor-γ t (ROR γ T). It competes with ROR γ T protein binding, thereby inhibiting Treg binding to DNA and promoting differentiation.67 Contrarily, some studies indicate that HIF-1α impairs the function and stability of Tregs.69 HIF-1α is known to facilitate FOXP3 proteasomal degradation and hinder the development of Tregs by altering T cell metabolism.70,71 Addressing this complexity, Miska established that hypoxia, rather than suppressing, actually promotes Treg migration through a Treg-specific ablation of HIF-1α in an animal model. In hypoxic conditions, HIF-1α diverts glucose away from mitochondria, making Tregs reliant on fatty acids for mitochondrial metabolism. This positions HIF-1α as a metabolic switch in Tregs, toggling between glycolytic-driven migration and oxidative phosphorylation-driven immunosuppression.71 In contrast to Tregs, TH17 cells predominantly represent an anti-inflammatory subset.72 As illustrated in Figure 2, HIF-1α acts as a crucial metabolic sensor, balancing Treg and TH17 cell dynamics. It modulates TH17 signature genes and amplifies TH17 development through the transcriptional activation of ROR γ T. ROR γ T and p300 congregate at the IL-17 promoter, forming a complex.67,73 Upon reaching a threshold expression level of ROR γ T, the cell transitions to a TH17 phenotype, potentially inciting inflammation.74
Hypoxia and B Cells
B cells, integral to adaptive immunity, exhibit varying responses to hypoxic environments, which remain incompletely understood. Research on mouse models has revealed that the germinal center (GC) micro-environment is hypoxic.75,76 Hypoxia has been observed to enhance B cell proliferation and promote class switch recombination (CSR) and plasmacyte differentiation.76 HIF-1α, a pivotal transcription factor, is crucial for the production of IL-6 and IL-10 in B cells. It binds to their promoters, augmenting their transcription.77,78 In patients with Rheumatoid Arthritis (RA), B cells are the primary source of IL-6 in peripheral blood and are closely associated with RA disease activity.77 The production of IL-10, which modulates the differentiation of innate-like B cells and B10 cells, leads to reduced IgM secretion.76 IL-10 is known to mitigate inflammation; conversely, IL-10-knockout mice have displayed chronic inflammation.79 Furthermore, the expression of hCXCR4 is vital in regulating B cell viability under hypoxia, influenced by hypoxia-induced ROS, HIF-1α, and Nrf2. CXCR4 also facilitates the migration of B-1a cells to the bone marrow, where they produce IgM antibodies.80 However, HIF-1α plays a role in the regeneration process post-pancreatitis by limiting B cell accumulation in the pancreas. Lee’s experiments showed that mice lacking pancreas-specific HIF-1α expression exhibited significantly impaired pancreatic regeneration, coupled with abnormal B cell accumulation in the pancreas following cerulein-induced pancreatitis.81
Influence Factors
Degree of Hypoxia
Hypoxia can be categorized based on its duration as either acute or chronic. Acute hypoxia typically arises during ARDS, acute cerebrovascular diseases, and cardiac arrest.82 In contrast, chronic hypoxia is more common in conditions such as chronic obstructive pulmonary disease (COPD), obstructive sleep apnea (OSA), and anemia. Recovery is often achievable following acute hypoxia, but chronic hypoxia tends to show a diminished recovery amplitude, attributed to specific molecular mechanisms that evolve with prolonged hypoxia.82 Hypoxia can also be classified according to its frequency into continuous or intermittent forms. Intermittent hypoxia is associated with a heightened inflammatory response compared to continuous hypoxia.83,84 Specifically, intermittent hypoxia notably escalates the activation of c-Jun and NF-κβ in M1-type macrophages, thereby fostering a pro-inflammatory phenotype.85 Moreover, the severity of hypoxia determines the extent of HIF-α activation in immune cells. Severe hypoxia can precipitate a bioenergetic crisis in cells, leading to necrosis. This cellular necrosis further exacerbates the inflammatory process by releasing cellular contents into the extracellular space.5
Body State
Various bodily conditions, such as age, obesity, and diabetes, significantly influence the degree of inflammation experienced under hypoxic conditions.86,87 Hypoxia activates related signaling pathways and interacts with aging pathways, potentially accelerating the aging process. In an aging state, two key developments occur. Firstly, aging-related inflammation leads to the accumulation of local inflammatory factors, potentially causing severe inflammation and even cytokine storms. Secondly, aging impairs various cellular functions, notably in epithelial cells and vascular smooth muscle cells, leading to frequent oxidative stress damage.86 Obesity induces a mild yet chronic inflammatory state within adipose tissue,87 driven by several pathways, including oxidative stress, endoplasmic reticulum stress, and adipose tissue hypoxia.87,88 Hypoxia acts as a principal trigger for adipokine dysregulation in obesity, promoting the expression of macrophage genes regulated by HIF-1α.89,90 Elevated expression and protein levels of HIF-1α have been observed in the adipose tissue of obese mice.91 Moreover, high glucose levels foster chronic inflammation and oxidative stress, elevating the expression of various pro-inflammatory cytokines.92,93 Zhao showed that the HIF-1α/JMJD1A signaling pathway is involved in oxidative stress and inflammation in human umbilical vein endothelial cells (HUVECs) that are caused by high glucose and low oxygen levels.92
The Anti-Inflammatory Therapies Under Hypoxia
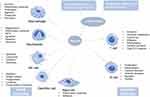
In the above study, we explored how hypoxia promotes the development of inflammation by influencing immune cells. Therefore, one of the effective strategies for the treatment of hypoxic inflammation is to regulate the activity of immune cells under hypoxic environment by targeting immune cells, so as to achieve the purpose of anti-inflammation and tissue regeneration. More information on immune cell targeting therapy strategies is summarized in Table 1.
 |
Table 1 Summary of Different Drugs Targeting Immune Cells |
Therapy in Macrophage
Numerous studies have shown that inhibiting HIF-1α steers macrophages towards an anti-inflammatory phenotype.95,100 Glucose metabolism significantly influences HIF activity. D-mannose elevates intracellular mannose-6-phosphate, hindering succinate-mediated HIF-1α activation.95 Similarly, Tan-IIA obstructs HIF-1α induction by reducing succinate levels.97 Drugs like Norisoboldine, Cynaroside, and Iminostilbene target the PKM2/HIF-1α axis. They decrease PKM2-HIF-1α binding and inhibit glycolysis-related proteins such as PFKFB3, HK2, and HIF-1α.96,98,100 Suppressing HIF-1α activation primarily influences macrophage polarization by modifying the release of anti-inflammatory cytokines, the glycolytic pathway balance, and NLRP3 inflammasome inhibition. Notably, IL-6 and TNF-α levels, M1 macrophage markers, decline with treatment. Conversely, IL-4 and IL-10 levels, which induce M2 macrophage polarization, increase significantly.10,114 Deng’s experiments revealed that BMSCs-derived exosomes regulate macrophage polarization by inhibiting glycolysis, with HIF-1α playing a crucial role in this anti-glycolysis effect.99 Tiliroside prevents classical M1 macrophage polarization by blocking glycolytic enzyme expression and reducing 2-NBDG uptake, a fluorescent deoxy-glucose analog.94 Therapies such as the co-assembly of hypoxia-sensitive macrocyclic amphiphiles with extracellular vesicles and ferulic acid induce the M1-to-M2 macrophage transition by inhibiting HIF-1α expression and the downstream NF-κβ signaling pathway.102,115 Beyond HIF-1α, the PPAR γ/STAT3 and GSK-3β/Nrf-2 pathways also regulate macrophage polarization.116–118 Curcumin, via the ERK signaling pathway, reduces HIF-1α levels, thereby lowering total cholesterol and lipid levels in macrophages and decreasing apoptosis.101 As highlighted, the NLRP3 inflammasome, crucial in inflammation development, is activated through the mTOR/HIF-1α/glycolysis pathway,119,120 Hydroxychloroquine impedes NLRP3 inflammasome activation via the NF-κβ signaling pathway.103
Therapy in Neutrophil
Anti-inflammatory drugs effectively suppress inflammation by inhibiting the release of inflammatory cytokines and reducing neutrophil infiltration, migration, and adhesion. HIF-1α, crucial for oxygen homeostasis under hypoxic conditions, is a significant target in this process. FG-4592 is a special small-molecule PHD inhibitor that stabilizes HIF-1α. This stops neutrophils from entering the cell, prevents cell damage, and reduces inflammation caused by low oxygen.105 Lu’s research indicated that Cyclosporine enhances neutrophil HIF-1α expression via the SIRT6–HIF‐1α–glycolysis axis. This elevation aids in fueling neutrophil glycolysis and the TCA cycle, reducing neutrophil migration, and alleviating severe ulcerative colitis.104 Moreover, targeting neutrophil adhesion is a vital therapeutic strategy. Sevoflurane diminishes neutrophil adhesion molecule expression and inhibits migration by stabilizing hypoxia-inducible factor 1α and the adenosine A2B receptor.106 In addition to the stabilizing effects of HIF-1α, research indicates that inhibitors of HIF-1α also play a crucial role in modulating neutrophil functions to mitigate inflammation. Itaconic acid, an endogenous product of macrophages, stimulates the transcription of the anti-inflammatory nuclear factor erythroid 2-related factor 2 (Nrf2). This activation leads to an upregulation of the cytoprotective enzyme heme oxygenase (HO-1) and concurrently reduces the release of pro-inflammatory cytokines.121 Gabriela discovered a derivative of itaconic acid, specifically 4-octyl itaconate (4-OI), which was introduced exogenously. This compound has been shown to inhibit the formation of NETs in mice. It achieves this by suppressing HIF-1α and activating HO-1.107 While the formation of neutrophil NETs is advantageous during the initial stages of infection, it becomes detrimental in subsequent inflammation stages caused by the infection.122 Additionally, YC-1, functioning as a HIF-1 inhibitor, effectively impedes the HMGB1/TLR4/NF-κβ signaling pathway. This inhibition subsequently reduces HIF-1α expression, curtailing the release of pro-inflammatory cytokines and the infiltration of neutrophils.108
Therapy in Adaptive Immunity
In the context of hypoxia, a key factor contributing to heightened inflammation is the imbalance between Treg and TH17 cells. Addressing this imbalance involves interventions at three levels: cytokines, receptors, and signaling pathways. TH17 cells, known for their pro-inflammatory actions, facilitate the release of cytokines such as TNF, IL-6, and IL-17. TNF inhibitors, including infliximab, etanercept, and adalimumab, are extensively utilized in treating rheumatoid arthritis.109 IL-17 inhibitors have demonstrated anti-inflammatory properties in both human and rat models of inflammatory diseases.67,113 Additionally, the TH17 transcription factor ROR γ t presents another target. Laura introduced SR1001, a high-affinity synthetic ligand, which inhibits TH17 cell differentiation and function by targeting ROR γ t.110 Hif-1α plays a crucial role in modulating the interplay between inflammation and T cells. Melatonin, an endogenous hormone, mitigates the expression of IL-17A and IFN-γ, thereby suppressing TH17 cells in experimental autoimmune uveitis mice through the oxidative stress/TXNIP/HIF-1α axis.112 Yao suggested that the HIF-1α inhibitor echinomycin improves Treg development by blocking the interaction between HIF-1α and Foxp3, and it weakens TH17 responses by blocking HIF-1α’s connection to the ROR γ t gene.111 STAT3 is crucial in triggering ROR γ t, a transcription factor specific to TH17 cells, which is essential for inducing the expression of IL17.123 Thus, Consequently, therapeutic strategies targeting the JAK-STAT signaling pathway are also frequently used in immune diseases and inflammatory diseases.123,124
Therapy Alleviating Hypoxic Micro-Environment Function
The previously discussed therapies focus on modulating immune cell responses to suppress inflammation induced by hypoxia. However, these do not directly address the hypoxic micro-environment itself. To counteract this, materials that either deliver or generate oxygen in hypoxic regions can mitigate hypoxic inflammation. This is achieved by alleviating anoxia, inhibiting anaerobic bacteria, and reducing ROS. Table 2 lists representative nanomaterials used to alleviate hypoxia.
 |
Table 2 Summary of Oxygenated Materials |
Oxygen Delivery in Hypoxic Regions
Currently, materials like perfluorocarbons (PFCs) and biomimetic nano-materials based on red blood cells are predominant in oxygen transport. To make these man-made oxygen carriers (AOCs), scientists use a variety of techniques, including electrospinning/electro-spraying, mechanical emulsification, SPG membrane emulsification, flow focusing, and the layer-by-layer technique with template particles.137
Perfluorocarbons
PFCs, with their unique ability to bind oxygen due to fluorine atoms replacing hydrogen, serve as efficient artificial oxygen carriers.138 Their capacity to dissolve significant amounts of oxygen stems from weak van der Waals forces, facilitating passive diffusion into oxygen-deprived zones.139 The dense electron clouds, elevated ionization potentials, and increased electron affinities in PFCs confer pronounced hydrophobicity and fluorophilicity. These properties, while characteristic of PFCs, present challenges in biological aqueous environments due to their inherent incompatibility with hydrophilic structures, unless appropriately conjugated with hydrophilic entities.125 Their oxygen release, which depends only on diffusion across concentration gradients, is noticeably inefficient.140 Additionally, PFCs can release metal ions, potentially leading to acute toxicity and oxidative damage in vital organs.141,142 Therefore, Patil introduced meth-acrylamide chitosan-modified perfluorocarbon chains (MACF) to create hydrogel dressings for dermal wounds. This hydrogel, as illustrated in Figure 3C, facilitates oxygen transport and enhances wound healing by modulating arginine and proline metabolism. Notably, it regulates M1 and M2 macrophage activation without macrophage phenotype polarization in wound tissues, with untreated wounds showing fewer macrophages.125 Additionally, oxygenating microgels were developed using PFC-modified chitosan through a water-in-oil mini-emulsion method and incorporated into 3D spheroid cultures to boost oxygen transport (Figure 3).126 Another good way to address the shortcomings of PFCs is to wrap them in nanoparticles. Yang synthesized perfluoro-decalin-encapsulated albumin nanoparticles within a hyaluronate gel, forming a nano-oxygenated gel. This gel effectively delivered oxygen to overcome local hypoxia and foster tissue regeneration, reducing macrophage density and inflammation.127 Moreover, Lee incorporated perfluorocarbon (PFC) nano-emulsions into a hydrogel dressing, enhancing its composition with epidermal growth factor (EGF)-loaded nanoparticles and poly-hexamethylene biguanide. This innovative blend was designed to simultaneously deliver anti-inflammatory and antimicrobial effects, as well as supplement oxygen. The experimental results demonstrated the hydrogel’s anti-inflammatory capabilities, attributed primarily to the release of chitosan molecules.128 This leads to a compelling proposition: combining the previously mentioned anti-inflammatory agents with PFCs to develop novel nanoparticles or hydrogels that play a multi-functional role.
 |
Figure 3 Perfluorocarbons deliver oxygen to hypoxia areas. (A) Representative PO2 vs time data for MACF initially saturated with oxygen (+O2) and MAC + O2 at RT. (B) Oxygen transport rate measurements. Superscript symbols “*” indicate p < 0.0001. (C) Arginine and proline metabolism pathway supplies fibrillary collagen synthesis. Reprinted from Acta Biomater, volume: 36, Patil PS, Fountas-Davis N, Huang H, et al. Fluorinated methacrylamide chitosan hydrogels enhance collagen synthesis in wound healing through increased oxygen availability. 164–174, Copyright 2016, with permission from Elsevier.125 (D) Illustration of radial oxygen measurements taken at various depths in control versus microgel loaded spheroids after 4 days (96 h) in culture. (E) Partial pressure of oxygen at various depths inside the spheroids. Reprinted from Patil PS, Mansouri M, Leipzig ND. Fluorinated Chitosan Microgels to Overcome Internal Oxygen Transport Deficiencies in Microtissue Culture Systems. Adv Biosyst. 2020; 4 (8): e1900250. © 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.126 |
Hemoglobin-Based Oxygen Carriers
Artificial materials often pose biocompatibility challenges and can elicit immune responses.143 In response, bionic materials, such as those derived from white or red blood cell membranes, are increasingly utilized.144 Red blood cells, notable for their double-concave structure and ability to deform mechanically, efficiently transport oxygen by forming oxy-hemoglobin.129,145 Guo capitalized on this by employing silica bio-replication to reconstruct red blood cells, embedding them with functional cargo such as hemoglobin (Hb) drugs and magnetic nanoparticles for enhanced oxygen transport.129 Hemoglobin consists of four peptide subunits, each containing a heme group. This group, featuring a porphyrin ring with centrally bound iron ions (Fe2+), is crucial for oxygen carriage. Oxygenated hemoglobin (HbO2) forms through reversible binding with oxygen. At mildly elevated temperatures, Hb acts as an efficient oxygen carrier, modulating its oxygen-binding ability for controlled release.130,146 Yang developed an injectable hydrogel combining hyaluronic acid-graft-dopamine (HA-DA) and polydopamine (PDA)-coated Ti3C2 Mxene nano-sheets. This formulation undergoes catalytic cross-linking via an oxyhemoglobin/hydrogen peroxide (HbO2/H2O2) system, activated by mild photothermal stimulation. HbO2 in this system releases oxygen in response to mild heat and captures atmospheric oxygen when near-infrared irradiation (NIR) is deactivated. HA-DA molecules in the hydrogel also help macrophages change from M1 to M2, which adds to its anti-inflammatory properties.130 However, hemoglobin molecules, when devoid of protective red blood cell membranes or biological environments, degrade quickly and lose oxygen transport efficiency. It’s been observed that modifying hemoglobin through polymerization, cross-linking, conjugation, encapsulation, or genetic recombination can enhance its stability and functionality.147 These modifications help reduce hemoglobin dissociation, prevent exosmosis of Hb, and maintain effective life cycles and transport capacities.148
Oxygen Production in situ in Hypoxic Areas
Catalase-Based Nanoparticles
Catalase, a vital endogenous antioxidant enzyme, plays a dual role. It not only acts as an antioxidant to eliminate ROS but also functions as an oxygen generator. This dual functionality is essential for oxygenating wound tissue, stimulating epithelial regeneration, fibroblast proliferation, migration, and angiogenesis, thereby facilitating tissue regeneration and wound healing.149 Zhou synthesized encapsulated S-methylisothiourea hemi-sulfate salt (SMT) and catalase (CAT) within ZIF-8 nanoparticles (NPs). These NPs, upon decomposition in the acidic endosomal environment, release CAT and SMT (Figure 4). SMT inhibits the release of inducible nitric oxide synthase (iNOS), thereby controlling mitochondrial respiration disruption caused by nitric oxide (NO). This regulation assists in moderating the release of H2O2 and ROS. Concurrently, CAT catalyzes the decomposition of excess H2O2 in the body into oxygen, further regulating mitochondrial respiration and suppressing HIF-1α expression.131 Building on this, researchers have proposed novel therapeutic strategies that integrate immune-targeted therapy with catalase. Han pioneered a combination of dendritic cell (DC)-targeted nanoplatforms with retinoic acid (RA) therapy, creating ZnO2/Catalase @liposome-Mannose nanoparticles (ZnCM NPs). These NPs modulate zinc and oxygen homeostasis; catalase within these NPs transforms H2O2 into oxygen, alleviating intracellular hypoxia. This assists Zn2+ in promoting the transition from DC to tolerogenic dendritic cells (tDCs), reducing immune damage in rheumatoid arthritis (RA).132 However, the concentration of H2O2 in wounds is limited, and its exogenous delivery is challenging to control, potentially exacerbating oxidative stress and causing toxicity. Scholars suggest a multi-enzyme cascade reaction approach, combining catalase with superoxide dismutase (SOD), to enhance ROS removal and oxygen production.150 SOD, a primary antioxidant enzyme, converts superoxide radicals to H2O2 and then to O2, acting as an antioxidant and anti-inflammatory agent.151 Yet the enzyme’s stability limits its application, which restricts its stable delivery in the body.152 Furthermore, catalase concentrations exceeding 500 U/mL may disrupt key cellular signaling molecules, posing potential cytotoxic risks.153 Therefore, optimizing catalase concentration to balance its oxygen-producing role in wound healing without inducing cytotoxicity is an area of ongoing research.
 |
Figure 4 Catalase-based nanoparticles produce oxygen in situ in hypoxia areas. (A) Schematic illustration of the synthesis processes for CAT&SMT@ZIF-8-Ab NPs. (B) Modified ZIF-8 NPs target synovial macrophages to regulate intracellular gases and reprogram the metabolic pathway, thus attenuating OA. (C) The percentages of M1 (CD206-negative and CD16/32-positive cells) and M2 (CD206-positive and CD16/32-negative cells cells) macrophages were evaluated by flow cytometry. (D) Expression levels of genes related to M1-type macrophage including IL-1β, IL-12b, IL-6, TNF-α, and Inos/Arg-1 were measured by Qrt-PCR. Superscript symbol “*” indicates comparisons with the first group, and superscript symbol “#” indicates comparisons with the second group. Comparisons between ZIF-8 and CAT&SMT@ZIF-8 NP-treated groups and CAT&SMT@ZIF-8 and CAT&SMT@ZIF-8-Ab NP-treated groups were calculated and marked with the superscript symbol “&”. Superscript symbols “*”, “#”, and “&” indicate p < 0.05; superscript symbols “**”, “##”, and “&&” indicate p < 0.01; NS means not significant. Reprinted from Zhou F, Mei J, Yang S, et al. Modified ZIF-8 Nanoparticles Attenuate Osteoarthritis by Reprogramming the Metabolic Pathway of Synovial Macrophages. ACS Appl Mater Interfaces. 2020; 12 (2): 2009–2022. Copyright 2022 American Chemical Society.131 |
Nano-Enzymes
Nano-particles exhibiting enzyme-like catalytic activity can alleviate hypoxia by catalyzing hydrogen peroxide (H2O2) into oxygen. Prominent nano-materials with such activity include Manganese dioxide (MnO2), Prussian blue (PB), Platinum (Pt), Fe3O4, etc.133,134,151,154 Platinum nanoparticles, in particular, can break the oxygen-oxygen bond of H2O2 to generate O2,155 demonstrating various oxidoreductase-like activities (type 2 NZ), including oxidase (OX), peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD).156 These nanoparticles also regulate ROS levels, impacting inflammatory signal transduction and pathways. Both in vitro and in vivo experiments have confirmed Pt nanoparticles’ potential anti-inflammatory effects, which mitigate phagocytic activation by clearing ROS-producing chambers and disrupting pathways involved in intracellular aggregation and pro-inflammatory gene transcription.156,157 However, the detailed mechanisms of their action in inflammation remain largely unexplored. Prussian blue nanoparticles stand out as promising catalysts. Tong utilized chlorin e6 (Ce6)-loaded Prussian blue nanoparticles for combating drug-resistant bacteria. These nanoparticles catalyze H2O2 into O2, alleviating the anaerobic bacterial environment. Concurrently, photosensitizers (PS) in the nanoparticles enhance the antibacterial effect by utilizing oxygen to generate significant amounts of ROS. Additionally, PB nanoparticles promote wound healing and anti-inflammatory effects by regulating macrophage polarization.152 MnO2 nano-sheets, a novel nano-enzyme, catalyze the abundant endogenous ROS (H2O2) into O2, presenting a promising solution for oxidative stress and hypoxia.134,158 Wang developed an injectable multifunctional hydrogel composed of ε-poly-lysine (EPL)-coated MnO2 nano-sheets (EM) and insulin-loaded, self-assembled aldehyde Pluronic F127 (FCHO) micelles. The hydrogel responds to elevated glucose levels in diabetes, which are associated with increased ROS production, impeding wound healing. MnO2 nano-sheets serve a dual function: clearing ROS and replenishing oxygen. When H2O2 breaks down EM nano-sheets, it starts a redox reaction that helps break down the hydrogel and speed up insulin release. This process not only scavenges ROS but also reduces glucose levels, thereby decreasing inflammation. Moreover, MnO2 nano-sheets have demonstrated excellent antibacterial properties.134
Metal Peroxides Decomposition Strategy
Metal peroxides, comprising metal ions and peroxide groups, interact with water to produce hydrogen peroxide (H2O2) and release metal ions. Current peroxide nano-systems primarily include copper peroxide (CuO2), calcium peroxide (CaO2), magnesium peroxide (MgO2), zinc peroxide (ZnO2), barium peroxide (BaO2), and titanium peroxide (TiOx).159 Among these, calcium peroxide is the most widely used in wound healing, owing to its optimal oxygen-producing potential and environmentally friendly by-products.12 Ma developed a pH-responsive O2 and H2O2 self-supplying zeolitic imidazolate framework-67 (ZIF-67) nano-system for photodynamic (PDT) and chemo-dynamic therapy (CDT) of wound infections. This system uses CaO2 to make oxygen and H2O2. The oxygen lowers hypoxia, which improves PDT caused by light from the outside, and H2O2, which is catalyzed by endogenous Co2+, makes hydroxyl radicals for CDT that is set off by Co2+.135 A major challenge with calcium peroxide is its rapid initial release of oxygen upon direct contact with water. Addressing this, Lim designed an oxygen-generating biomaterial using calcium peroxide, coated with catalase (which quickly decomposes H2O2 into water and oxygen), and hydrophobic beeswax (BW) for prolonged oxygen release. Experiments demonstrated that the beeswax addition extended the oxygen burst duration from 2 to 12 hours.136 However, the decomposition by-products of calcium peroxide may cause olfactory disorders, and its direct exposure to the lower respiratory tract can lead to pulmonary edema. Therefore, the bio-safety of calcium peroxide warrants further enhancement.136
Conclusion
Immune cells play a crucial role in the interplay between hypoxia and inflammation. While targeting hypoxic signaling pathways, there is a potential trade-off between the regulatory and pro-inflammatory functions of immune cells. As shown in Figure 5, understanding how hypoxia exacerbates inflammation via immune cells is essential for effectively treating hypoxia-induced inflammatory diseases. This paper has reviewed existing drugs and materials that target immune cells for the treatment and mitigation of hypoxia-induced inflammation, paving the way for new therapeutic targets. The paper also highlights the significance of bio-active nano-materials capable of delivering or producing oxygen in situ at infected sites. These materials, known for reducing hypoxia and clearing ROS, also influence immune cell functionality. Therefore, the development of bio-active materials must be informed by a thorough understanding of their underlying mechanisms. Strategically selecting and combining these bio-active materials from a mechanistic viewpoint can lead to the design of novel, multifunctional materials. Such an approach promises more effective prevention and treatment of hypoxic inflammation.
Data Sharing Statement
No data was used for the research described in the article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.

No comments:
Post a Comment