My God, you're that clueless that you think reviews do one fucking thing to get survivors recovered? Do some real research and create EXACT REHAB PROTOCOLS! I'd fire you all.
Literature review of stroke assessment for upper-extremity physical function via EEG, EMG, kinematic, and kinetic measurements and their reliability
Journal of NeuroEngineering and Rehabilitation volume 20, Article number: 21 (2023)
Abstract
Background
Significant clinician training is required to mitigate the subjective nature and achieve useful reliability between measurement occasions and therapists. Previous research supports that robotic instruments can improve quantitative biomechanical assessments of the upper limb, offering reliable and more sensitive measures. Furthermore, combining kinematic and kinetic measurements with electrophysiological measurements offers new insights to unlock targeted impairment-specific therapy. This review presents common methods for analyzing biomechanical and neuromuscular data by describing their validity and reporting their reliability measures.
Methods
This paper reviews literature (2000–2021) on sensor-based measures and metrics for upper-limb biomechanical and electrophysiological (neurological) assessment, which have been shown to correlate with clinical test outcomes for motor assessment. The search terms targeted robotic and passive devices developed for movement therapy. Journal and conference papers on stroke assessment metrics were selected using PRISMA guidelines. Intra-class correlation values of some of the metrics are recorded, along with model, type of agreement, and confidence intervals, when reported.
Results
A total of 60 articles are identified. The sensor-based metrics assess various aspects of movement performance, such as smoothness, spasticity, efficiency, planning, efficacy, accuracy, coordination, range of motion, and strength. Additional metrics assess abnormal activation patterns of cortical activity and interconnections between brain regions and muscle groups; aiming to characterize differences between the population who had a stroke and the healthy population.
Conclusion
Range of motion, mean speed, mean distance, normal path length, spectral arc length, number of peaks, and task time metrics have all demonstrated good to excellent reliability, as well as provide a finer resolution compared to discrete clinical assessment tests. EEG power features for multiple frequency bands of interest, specifically the bands relating to slow and fast frequencies comparing affected and non-affected hemispheres, demonstrate good to excellent reliability for populations at various stages of stroke recovery. Further investigation is needed to evaluate the metrics missing reliability information. In the few studies combining biomechanical measures with neuroelectric signals, the multi-domain approaches demonstrated agreement with clinical assessments and provide further information during the relearning phase. Combining the reliable sensor-based metrics in the clinical assessment process will provide a more objective approach, relying less on therapist expertise. This paper suggests future work on analyzing the reliability of metrics to prevent biasedness and selecting the appropriate analysis.
Background
Stroke is one of the leading causes of death and disability in developed countries. In the United States, a stroke occurs every 40 s, ranking stroke as the fifth leading cause of death and the first leading cause of disability in the country [1]. The high prevalence of stroke, coupled with increasing stroke survival rates, puts a growing strain on already limited healthcare resources; the cost of therapy is elevated [2] and restricted mostly to a clinical setting [3], leading to 50% of survivors that reach the chronic stage experiencing severe motor disability for upper extremities [4]. This highlights the need for refined (improved) assessment which can help pair person-specific impairment with appropriately targeted therapeutic strategies.
Rehabilitation typically starts with a battery of standardized tests to assess impairment and function. This initial evaluation serves as a baseline of movement capabilities and usually includes assessment of function during activities of daily living (ADL). Because these clinical assessments rely on trained therapists as raters, the scoring scale is designed to be discrete and, in some cases, bounded. While this improves the reliability of the metric [5] (i.e., raters more likely to agree), it also reduces the sensitivity of the scale. Furthermore, those assessment scales that are bounded, such as the Fugl-Meyer Assessment (FMA) [6], Ashworth or Modified Ashworth (MA) Scale [7], and Barthel Index [8], suffer from floor/ceiling effects where the limits of the scales become insensitive to the extremes of impairment and function. It is therefore important to develop new clinical assessment methods that are objective, quantifiable, reliable, and sensitive to change over the full range of function and impairment.
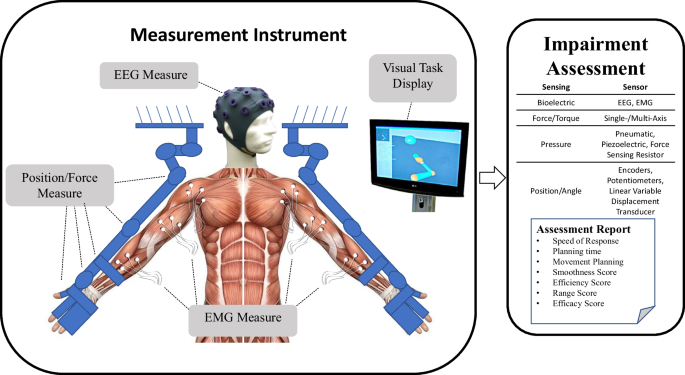
Over the last several decades, robotic devices have been designed and studied for administering post-stroke movement therapy. These devices have begun being adopted into clinical rehabilitation practice. More recently, researchers have proposed and studied the use of robotic devices to assess stroke-related impairments as an approach to overcome the limitations of existing clinical measures previously discussed [9,10,11,12]. Robots may be equipped with sensitive measurement devices that can be used to rate the person’s performance in a predefined task. These devices can include measuring kinematic (position/velocity), kinetic (force/torque), and/or neuromuscular (electromyography/electroencephalography) output from the subject during the task. Common sensor-based robotic metrics for post-stroke assessment included speed of response, planning time, movement planning, smoothness, efficiency, range, and efficacy [13, 14]. Figure 1 demonstrates an example method for comprehensive assessment of a person who has suffered a stroke with data acquired during robotically administered tests. Furthermore, there is potential for new and more comprehensive knowledge to be gained from a wider array of assessment methods and metrics that combine the benefits of biomechanical (e.g., kinematic and kinetic) and neurological (e.g., electromyographic and electroencephalographic) measures [15,16,17,18,19,20,21,22].
Biomechanical assessment
Many classical methods of assessing impairment or function involve manual and/or instrumented quantification of performance through measures of motion (i.e., kinematic) and force (i.e., kinetic) capabilities. These classical methods rely on the training of the therapist to evaluate the capabilities of the person through keen observation (e.g., FMA [6] and MA [7]). The quality of kinematic and kinetic measures can be improved with the use of electronic-based measurements [23]. Robotic devices equipped with electronic sensors have the potential to improve the objectivity, sensitivity, and reliability of the assessment process by providing a means for more quantitative, precise, and accurate information [9,10,11,12, 24,25,26,27,28]. Usually, the electronic sensors on a rehabilitation robotic device are used for control purposes [29,30,31]. Robotics can also measure movement outputs, such as force or joint velocities, which the clinician may not be able to otherwise measure as accurately (or simultaneously) using existing clinical assessment methods [23]. With accurate and repeatable measurement of forces and joint velocities, sensor-based assessments have the potential to assess the person’s movement in an objective and quantifiable way. This article reviews validity and reliability of biomechanical metrics in relationship to assessment of motor function for upper extremities.
More at link.
No comments:
Post a Comment