So is this the reason that leg wraps and leg compressions reduce stroke severity? Has your hospital implemented them yet? WHAT THE FUCK ARE THEY WAITING FOR?
leg compressions (16 posts to September 2015)
leg wraps (7 posts to May 2013)
The latest here:
Emerging Role of microRNAs in Stroke Protection Elicited by Remote Postconditioning
- Division of Pharmacology, Department of Neuroscience, School of Medicine, “Federico II” University of Naples, Naples, Italy
Remote ischemic conditioning (RIC) represents an innovative and attractive neuroprotective approach in brain ischemia. The purpose of this intervention is to activate endogenous tolerance mechanisms by inflicting a subliminal ischemia injury to the limbs, or to another “remote” region, leading to a protective systemic response against ischemic brain injury. Among the multiple candidates that have been proposed as putative mediators of the protective effect generated by the subthreshold peripheral ischemic insult, it has been hypothesized that microRNAs may play a vital role in the infarct-sparing effect of RIC. The effect of miRNAs can be exploited at different levels: (1) as transducers of protective messages to the brain or (2) as effectors of brain protection. The purpose of the present review is to summarize the most recent evidence supporting the involvement of microRNAs in brain protection elicited by remote conditioning, highlighting potential and pitfalls in their exploitation as diagnostic and therapeutic tools. The understanding of these processes could help provide light on the molecular pathways involved in brain protection for the future development of miRNA-based theranostic agents in stroke.
Introduction
Ischemic conditioning is a neuroprotective approach able to make the brain more resistant to an ischemic insult through the exposure to a subthreshold stimulus. This method provides neuroprotection when the conditioning stimulus is administered either before or after the detrimental ischemia, i.e., preconditioning or postconditioning.
Indeed, ischemic preconditioning is an endogenous defensive process triggered by a subclinical ischemic event that increases tissue resilience or, in other words, organ resistance to a subsequent, typically dangerous, ischemia episode. Non-ischemic conditioning cues can also promote neuroprotection against an ischemic insult, a phenomenon known as “cross-protection”(1). Surprisingly, when the subliminal boost is delivered after the ischemia insult, the neuroprotection achieved, referred to as postconditioning, is comparable to that shown in ischemic preconditioning models. Remarkably, combining preconditioning and postconditioning does not result in greater protection than either treatment alone (2).
We define preconditioning, perconditioning, and postconditioning from a strictly temporal standpoint, depending on whether the conditioning stimulus is delivered before, during, or after the detrimental ischemia (Figure 1).
It is now well recognized that stressing preconditioning or postconditioning stimuli elicit multiple endogenous defensive mechanisms in the brain, resulting in a latent protective phenotype. When the lethal ischemic insult is delivered inside this inactive protective phenotype, a partitioned set of reactions are triggered that are strikingly different from the phenotype of the unprimed or non-preconditioned brain, resulting in the so-called ischemia-tolerant phenotype (3).
Surprisingly enough, in the last years we and others produced data supporting the idea that preconditioning and postconditioning exert their effects also when applied to an anatomical site distant from the brain (4–8). In fact, remote ischemic conditioning (RIC) represents an innovative and attractive protective approach in brain ischemia. This method is designed to elicit the initiation of endogenous tolerance processes by providing a not-dangerous ischemic event in a distant tissue, i.e., arms or limbs, leading to a protective systemic response against stroke. Several studies have examined the effectiveness of RIC in reducing the effects of ischemic brain injury, as well as the potential pathophysiological pathways involved (4–8).
Among the multiple candidates that have been proposed as putative mediators of the protective effect generated by the subthreshold peripheral ischemic insult, it has been hypothesized that also in the case of remote conditioning, as it occurs in the case of direct conditioning (9–13), miRNAs may play a vital role in the infarct sparing effect (5, 7).
The effect of miRNAs during RIC can be exploited at different levels: (a) as transducers of protective messages to the brain or (b) as effectors of brain protection.
This review will summarize the most recent evidence supporting the involvement of microRNAs in the protection of the brain caused by remote conditioning, highlighting the potential and pitfalls in their exploitation as diagnostic and therapeutic tools.
More at link.
 Giuseppe Pignataro
Giuseppe Pignataro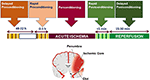
No comments:
Post a Comment