Interesting, but are these other methods to increase blood flow better?
Your doctor should be doing something from one of these.
blood flow (73 posts)
brain blood flow (1)
cerebral blood flow (15)
Cerebral Blood Flow Velocity (1)
Or is it more important to deliver more oxygen to your brain?
Possible solutions: Obviously not vetted coming from me. Don't do them.
Normobaric oxygen (10)
How to Improve Your Brain Function with An Oxygen Concentrator April 2018
Or is it more important to increase the loading ability of red blood cells to carry more oxygen?
Like this?
University of Glasgow Study Demonstrates the Ability of Oxycyte® to Supply Oxygen to Critical Penumbral Tissue in Acute Ischemic Stroke August 2012
Or like this?
chronic cannabis users have higher cerebral blood flow and extract more oxygen from brain blood flow than nonusers. August 2017
The latest here:
Stimulating the Facial Nerve to Treat Ischemic Stroke: A Systematic Review
- 1Department of Neurosurgery, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 2Sinai BioDesign, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 3The Grove School of Engineering, The City College of New York, New York, NY, United States
- 4Department of Otolaryngology, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Acute ischemic stroke (AIS) is a common devastating disease that has increased yearly in absolute number of cases since 1990. While mechanical thrombectomy and tissue plasminogen activator (tPA) have proven to be effective treatments, their window-of-efficacy time is very short, leaving many patients with no viable treatment option. Over recent years there has been a growing interest in stimulating the facial nerves or ganglions to treat AIS. Pre-clinical studies have consistently demonstrated an increase in collateral blood flow (CBF) following ganglion stimulation, with positive indications in infarct size and neurological scores. Extensive human trials have focused on trans-oral electrical stimulation of the sphenopalatine ganglion, but have suffered from operational limitations and non-significant clinical findings. Regardless, the potential of ganglion stimulation to treat AIS or elongate the window-of-efficacy for current stroke treatments remains extremely promising. This review aims to summarize results from recent trial publications, highlight current innovations, and discuss future directions for the field. Importantly, this review comes after the release of four important clinical trials that were published in mid 2019.
Introduction
Acute Ischemic Stroke
Stroke is the leading cause of disability and the fifth leading cause of death in the United States (US). Approximately 795,000 people experience a new or recurrent stroke each year (1). Acute ischemic stroke (AIS) occurs when an obstruction within a blood vessel decreases cerebral blood flow, depriving nerve cells of oxygen and leading to severe metabolic failure and neural death (2–4). Immediately following stroke, a section of the brain referred to as the ischemic core is subject to extreme hypoxia, leading to irreversible brain damage (5). The area surrounding the ischemic core, the ischemic penumbra, is severely hypoperfused and non-functioning, yet can regain functionality if blood flow is restored to the area (5, 6). This recovery is highly time-dependent, as the penumbra rapidly evolves into the ischemic core (6, 7). The recovery of the penumbra has been demonstrated to have a significant effect on clinical outcomes; Meretoja et al. showed that for every 20-min reduction in time to reperfusion increases the average disability-free life span by 3 months (8).
Current Treatments: Endovascular Thrombectomy and Tissue Plasminogen Activator
Management for AIS relies on rapid treatment times to avoid penumbra evolution. The goal of modern stroke treatment facilities is to reperfuse the ischemic area via endovascular thrombectomy, mechanically removing the blood clot with catheter-based devices (9). The faster the patient achieves reperfusion, the more likely the patient will have excellent neurological outcomes at 90 days (10). The second treatment paradigm for AIS is the use of intravenous (IV) tissue plasminogen activator (tPA), which acts to chemically break down clots (11–14). IV-tPA is commonly used prior to patient transport for thrombectomy.
Both endovascular thrombectomy and IV-tPA suffer from a limited window-of-efficacy. Currently, stroke guidelines list the acceptable window of treatment for mechanical thrombectomy at 24 h (15, 16), and a recommended IV-tPA door-to-treatment time of 60 min (10, 17, 18). These windows are frequently missed, with fewer than a third of patients in the U.S. treated within the IV-tPA 60 min window (19). Mandatory neuroimaging, presence of a highly-trained neurointerventionalist, and hospital transfer times all reduce event-to-treatment times and result in reductions of successful functional outcomes following recanalization (16, 20, 21).
Thrombectomy is a well-established treatment (15, 16, 22), but patients routinely fail to achieve functional independence (mRS ≤ 2) due to extensive event-to-treatment times (15, 16). There is a clear need for innovative approaches to extend the window-of-efficacy for endovascular thrombectomy and IV-tPA.
Time Is Brain: Inhibiting the Evolution of the Ischemic Penumbra
Researchers have recently sought to find new approaches to arrest the evolution of the ischemic penumbra and keep this susceptible region from becoming irreversibly damaged. Current approaches aim to either enhance oxygen delivery to the penumbra or reduce tissue oxygen demand (6). In addition to door-to-treatment time limitations, many patients also become ineligible for mechanical thrombectomy due to large ischemic core volumes. Inhibiting the evolution of the penumbra may help buy time by limiting core volume growth from reaching recommended exclusionary levels (23, 24). Inhibition of penumbra evolution could also be highly beneficial when combined with IV-tPA, potentially increasing its effectiveness and elongating its window-of-efficacy (6).
Increased Collateral Blood Circulation Through Ganglion Stimulation
Facial nerve-induced vasodilation of the cerebral arteries is an emerging therapeutic target that seeks to increase collateral blood flow in ischemic brain tissue, improve oxygen availability, and improve patient functional outcomes after stroke. Collateral circulation refers to alternative, pre-existing vascular pathways that deliver blood to target tissue when the primary vessel is occluded (25). Imaging of the brain and vessels has shown that collateral blood flow can preserve brain tissue for hours after major arteries to the brain are blocked (26). Figure 1 illustrates how facial nerve-induced increased collateral blood flow has the ability to ameliorate clot-induced tissue death by limiting ischemic penumbra evolution.
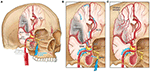
Figure 1. Increased collateral blood circulation by sphenopalatine ganglion (SPG) stimulation. (A) Anatomical overview of stroke affected brain region before SPG stimulation. (B) The SPG contains parasympathetic fibers that synapse in the ganglion and innervate the internal carotid artery (ICA) through the deep petrosal nerve. (C) SPG stimulation induces vasodilation of blood vessels in the anterior circulation, increasing blood flow to the affected area limiting the evolution of the penumbra and reducing infarct volume.
The sphenopalatine ganglion (SPG), also known as the pterygopalatine ganglion, is the largest and most superior ganglion of the sympathetic and parasympathetic nervous system and contains the largest collection of neurons in the calvarium outside of the brain (27). Humans have two SPGs, on each side of the midface, located within the viscerocranium in a space called the pterygopalatine fossa. This fossa has direct connections to the middle cranial fossa, nasal cavity, orbit, infratemporal fossa and oral cavity (28, 29). The SPG contains parasympathetic fibers that synapse in the ganglion and innervate the internal carotid artery (ICA) through the deep petrosal nerve (30). Electrical stimulation of the SPG activates the fibers, releasing several neurotransmitters such as acetylcholine, vasoactive intestinal polypeptide, peptide histidine isoleucine, and nitrous oxide that play a role in inducing vasodilation of blood vessels in the anterior circulation (26, 31).
The geniculate ganglion is a small collection of somatosensory and gustatory ganglion cells (32) located within the temporal bone along the axis of the ear canal. Similar to the SPG, the geniculate ganglion has parasympathetic connections to cerebral arteries and has also been demonstrated to increase cerebral blood flow (33, 34). Its curved shape allows for greater susceptibility to be activated location makes it easier to access through non-invasive routes (33, 35).
More at link.
 Turner S. Baker
Turner S. Baker Justin Robeny1,2†,
Justin Robeny1,2†,  Danna Cruz
Danna Cruz
No comments:
Post a Comment