Nothing on how to prevent this iron in the brain or even how it got there. So for right now useless information. I take an iron pill daily right before donating blood so I don't fall below that hemoglobin test.
Maybe this?
Diet affects brain iron levels differently in men and women, UB pilot study shows
The latest here:
Newfound Link Between Alzheimer’s and Iron Could Lead to New Medical Interventions
Summary: A new imaging probe revealed an increase in iron redox in brain areas where amyloid plaques occur in the brain in Alzheimer’s disease.
Source: UT Austin
There is a growing body of evidence that iron in the brain may play a role in Alzheimer’s disease.
Lending weight to that idea, a new imaging probe has for the first time shown that in the same regions of the brain where the amyloid beta plaques associated with Alzheimer’s occur, there is also an increase in iron redox, meaning the iron in these regions is more reactive in the presence of oxygen.
Their imaging probe could yield even more details about the causes of Alzheimer’s and help in the search for new drugs to treat it.
A team from The University of Texas at Austin and the University of Illinois at Urbana-Champaign published a study today on the new imaging technique and findings in Science Advances.
“The link between iron redox and Alzheimer’s disease has been a black box,” said Yi Lu, corresponding author and professor of chemistry at UT Austin. “The most exciting part to me is that we now have a way to shine light into this black box so that we can begin to understand this whole process in much more detail.”
About a decade ago, scientists discovered ferroptosis, a process in the body that is dependent on elevated iron levels, leads to cell death and plays a key role in neurodegenerative diseases, such as Alzheimer’s.
Using magnetic resonance imaging on living Alzheimer’s patients, scientists have observed that these patients tend to have elevated iron levels in the brain, although that method doesn’t differentiate between different forms of iron.
Together, these findings suggested that iron might play a role in destroying brain cells in Alzheimer’s patients.
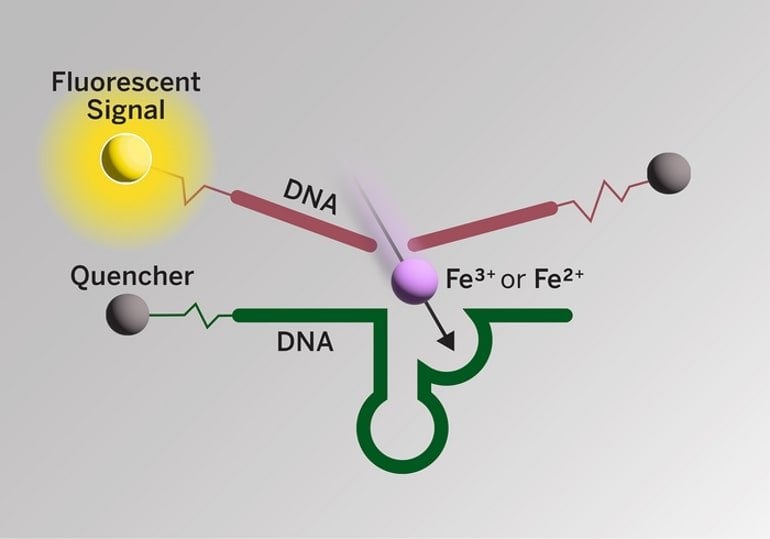
For the new study, the researchers developed DNA-based fluorescent sensors that can detect two different forms of iron (Fe2+ and Fe3+) at the same time in cell cultures and in brain slices from mice genetically modified to mimic Alzheimer’s. One sensor glows green for Fe2+ and the other glows red for Fe3+. This is the first imaging technique that can simultaneously detect both forms of iron in cells and tissue while also indicating their quantity and spatial distribution.
“The best part about our sensor is that we can now visualize the changes of Fe2+ and Fe3+ and their ratios in each location,” said Yuting Wu, a co-first author of the study and a postdoctoral researcher in Lu’s lab at UT Austin. “We can change one parameter at a time to see if it changes the plaques or the oxidative states of iron.”
That ability could help them better understand why there is an increased ratio of Fe3+ to Fe2+ in the location of amyloid beta plaques and whether increased iron redox is involved in forming the plaques.
Another key question is whether the iron redox is directly involved in cell death in Alzheimer’s, or simply a byproduct. The researchers plan to explore this question in Alzheimer’s mice. If further research determines that iron and its redox changes indeed cause cell death in Alzheimer’s patients, that information could provide a potential new strategy for drug development.
In other words, perhaps a drug that change the ratio Fe3+ to Fe2+ could help protect brain cells. The new imaging probe could be used to test how well drug candidates work at changing the ratio.
To develop the sensors, the scientists first hired a commercial lab to produce a library of 100 trillion short DNA strands, through a chemical process called oligonucleotide synthesis.
They then conducted a screening process to find those strands that recognize — or in chemistry parlance “bind tightly to and conduct a catalytic reaction with” — a specific form of iron and not any other forms.
To complete the sensors, other components were added including molecules called fluorophores that glow in a specific color when the probe recognizes the specific form of iron.
Lu, who moved his lab to UT Austin from the University of Illinois at Urbana-Champaign in the summer of 2021, collaborated with researchers there including professor of chemistry Liviu Mirica.
Funding: This work was supported by the National Institutes of Health, the Alzheimer’s Association and the Robert A. Welch Foundation. Lu holds the Richard J.V. Johnson – Welch Regents Chair in Chemistry.
About this Alzheimer’s disease research news
Author: Marc Airhart
Source: UT Austin
Contact: Marc Airhart – UT Austin
Image: The image is credited to David Steadman/University of Texas at Austin
Original Research: Open access.
“Simultaneous Fe2+/Fe3+ imaging shows Fe3+ over Fe2+ enrichment in Alzheimer’s disease mouse brain” by Yi Lu et al. Science Advances
Abstract
Simultaneous Fe2+/Fe3+ imaging shows Fe3+ over Fe2+ enrichment in Alzheimer’s disease mouse brain
Visualizing redox-active metal ions, such as Fe2+ and Fe3+ ions, are essential for understanding their roles in biological processes and human diseases. Despite the development of imaging probes and techniques, imaging both Fe2+ and Fe3+ simultaneously in living cells with high selectivity and sensitivity has not been reported.
Here, we selected and developed DNAzyme-based fluorescent turn-on sensors that are selective for either Fe2+ or Fe3+, revealing a decreased Fe3+/Fe2+ ratio during ferroptosis and an increased Fe3+/Fe2+ ratio in Alzheimer’s disease mouse brain.
The elevated Fe3+/Fe2+ ratio was mainly observed in amyloid plaque regions, suggesting a correlation between amyloid plaques and the accumulation of Fe3+ and/or conversion of Fe2+ to Fe3+.
Our sensors can provide deep insights into the biological roles of labile iron redox cycling.
No comments:
Post a Comment