Well then roll this out to all 10 million yearly stroke survivors so they can inform their medical staff of this. And then the medical staff can implement the EXACT STROKE REHAB PROTOCOLS that fix the impairments found.
Reliable and valid robot-assisted assessments of hand proprioceptive, motor and sensorimotor impairments after stroke
Journal of NeuroEngineering and Rehabilitation volume 18, Article number: 115 (2021)
Abstract
Background
Neurological injuries such as stroke often differentially impair hand motor and somatosensory function, as well as the interplay between the two, which leads to limitations in performing activities of daily living. However, it is challenging to identify which specific aspects of sensorimotor function are impaired based on conventional clinical assessments that are often insensitive and subjective. In this work we propose and validate a set of robot-assisted assessments aiming at disentangling hand proprioceptive from motor impairments, and capturing their interrelation (sensorimotor impairments).
Methods
A battery of five complementary assessment tasks was implemented on a one degree-of-freedom end-effector robotic platform acting on the index finger metacarpophalangeal joint. Specifically, proprioceptive impairments were assessed using a position matching paradigm. Fast target reaching, range of motion and maximum fingertip force tasks characterized motor function deficits. Finally, sensorimotor impairments were assessed using a dexterous trajectory following task. Clinical feasibility (duration), reliability (intra-class correlation coefficient ICC, smallest real difference SRD) and validity (Kruskal-Wallis test, Spearman correlations
with Fugl-Meyer Upper Limb Motor Assessment, kinesthetic Up-Down Test, Box & Block Test) of robotic tasks were evaluated with 36 sub-acute stroke subjects and 31 age-matched neurologically intact controls.
Results
Eighty-three percent of stroke survivors with varied impairment severity (mild to severe) could complete all robotic tasks (duration: <15 min per tested hand). Further, the study demonstrated good to excellent reliability of the robotic tasks in the stroke population (ICC>0.7, SRD<30%), as well as discriminant validity, as indicated by significant differences (p-value<0.001) between stroke and control subjects. Concurrent validity was shown through moderate to strong correlations (
=0.4-0.8) between robotic outcome measures and clinical scales. Finally, robotic tasks targeting different deficits (motor, sensory) were not strongly correlated with each other (0.32, p-value>0.1), thereby presenting complementary information about a patient’s impairment profile.
Conclusions
The proposed robot-assisted assessments provide a clinically feasible, reliable, and valid approach to distinctly characterize impairments in hand proprioceptive and motor function, along with the interaction between the two. This opens new avenues to help unravel the contributions of unique aspects of sensorimotor function in post-stroke recovery, as well as to contribute to future developments towards personalized, assessment-driven therapies.
Background
At the level of the hand, somatosensory and motor function, as well as the interplay between the two, are essential for performing dexterous and skillful movements during activities of daily living (ADLs) [1,2,3,4]. For example when grasping a small object, proprioception is necessary to sense the current position of the limb [5, 6]. This sensory input is then integrated by the central nervous system to shape the motor output, a process called sensorimotor integration [7, 8]. Subsequently, the motor system is responsible for eliciting and executing the planned movement [9].
Neurological injuries such as stroke often disrupt specific aspects of this process, which consequently prevents affected individuals from performing ADLs [10, 11]. Often the exact impairments that cause activity limitations are unclear, although their detection would be a prerequisite to designing appropriate rehabilitation strategies tailored to each patient’s impairment profile [12]. Most commonly reported are motor impairments, with 80% of stroke survivors experiencing paresis [13,14,15,16]. However, some activity limitations that seem to originate from a motor function impairment may be caused by disturbed proprioceptive feedback [7]. Somatosensory function is in fact frequently affected and has been shown to be associated with poor functional recovery and higher activity limitations, although the reporting prevalence varies between 23 and 67% [17,18,19,20,21,22].
The difficulty in accurately identifying each patients’ impairment profile originates, among others, from the lack of sensitive assessment methods [23, 24]. Most widely used clinical assessments are observer-based and subjective, not optimal for providing reproducible stimuli, and prone to floor/ceiling effects [24, 25]. Further, many clinical methods focus on evaluating activity limitations (e.g. Action Research Arm Test, Box & Block Test [26, 27]), however there is a lack of tools that could help in understanding the underlying cause of decreased performance. Existing clinical assessments provide only a global measure of impairments (e.g. Fugl-Meyer Upper Limb Assessment [23]) and multiple assessments are needed to holistically evaluate sensorimotor impairment profiles, hence they are rarely performed at regular time intervals throughout rehabilitation [28]. As clinical methods typically do not assess somatosensory, motor and sensorimotor impairments through a single, standardized assessment setup, it is difficult to systematically compare those impairment modalities and understand how they change over time.
Technology-driven solutions provide a promising complement to conventional clinical assessments [1, 12, 29]. Robot-assisted methods are objective (not relying on observer judgement), accurate (e.g. able to measure exact body position/force applied), as well as capable of delivering precise, reproducible stimuli (e.g. to assess sensory function or spasticity [30, 31]). Further, it becomes possible to evaluate different impairments with one single device through multiple robot-assisted assessment tasks, which results in a time-efficient and more comprehensive overview of impairments. This also allows to compare different impairment modalities (e.g. motor and sensory) with each other in a standardized way, potentially providing new insights into upper limb impairment profiles. Even though they are promising, the existing robotic approaches aiming at concurrent sensory and motor assessment of the hand remain in their infancy. The methods proposed so far focus on proximal joints of the upper limb [32, 33], consist of tasks that target only a specific impairment modality (e.g. proprioception, without the possibility to concurrently assess motor impairment) [34,35,36,37], or fail to provide a detailed evaluation of clinimetric properties of their outcome measures (reliability, measurement error, validity) [12, 38]. Reporting of test-retest reliability and measurement error is essential to understand the sensitivity of an assessment metric to capture different impairments and detect changes over time [38], while the study of concurrent validity is important to relate a new technological approach to the commonly accepted assessment methods [39]. The current lack of standardized evaluations of reliability and validity in the target population makes new assessment technologies less likely to be clinically accepted and applied outside of research projects [40].
The objective of this work was to propose and evaluate a new set of assessments of hand proprioceptive, motor and sensorimotor impairments, implemented on a single, previously described robotic platform (ETH MIKE: Motor Impairment and Kinesthetic Evaluation) [41, 42]. This one degree-of-freedom end-effector device can provide well-controlled movement stimuli to the index finger metacarpophalangeal (MCP) joint and sensitively measure its kinematic and kinetic responses. The index finger was selected due to its relevance in many ADLs (grasping, precision grip [43]). Furthermore, the ability to actively extend the MCP joint is often presented as an early predictor of functional recovery, as it is related to the degree of sparing of cortico-motoneuronal pathways after stroke [44, 45]. From a practical perspective, focusing on a single joint allows to simplify the technology, which increases clinical usability. In this paper we propose a battery of five behavioural tasks and their outcome measures, three of which address motor impairments, one targets proprioception and one measures combined sensorimotor deficits. We investigate the reliability and validity of these robot-assisted assessments in a group of 36 participants with stroke and in an age-matched group of 31 neurologically intact controls. We hypothesized that the newly proposed robot-assisted assessment metrics (i) are reliable due to the objective nature of the tasks, their repeatability and the standardized protocol; (ii) allow to distinguish stroke patients from control subjects and identify different impairment profiles; (iii) can separately quantify proprioceptive, motor and sensorimotor impairments and correlate with corresponding clinical scales.
This work aspires to contribute to the field of neurorehabilitation by providing novel objective assessments, which aim at disentangling different aspects of sensorimotor impairments in order to better understand the cause of observed activity limitations. In the long term the proposed robot-assisted assessments intend to help in designing more effective therapies, as well as in tracking and predicting recovery of patients after neurological injuries.
Methods
Subjects
Thirty-six participants with stroke were recruited for this study among the patients receiving an inpatient neurological rehabilitation at the Kliniken Schmieder Allensbach, Germany. Inclusion criteria were: above 18 years old, diagnosis of stroke (ischemic or hemorrhagic), and the ability to passively move the subject’s MCP joint by at least
. Exclusion criteria were: inability to understand instructions and pain when moving the MCP joint. Moreover, we designed the study to include a maximum of 40% of subjects with intact proprioception as measured by a conventional clinical scale. This design choice was made to allow for validating the newly proposed measure of proprioception. In addition, thirty-one age-matched neurologically intact control subjects were recruited. The inclusion criteria for this group were: right-handed and above 50 years old. The exclusion criteria was any history of neurological, orthopaedic or rheumatologic disease affecting wrist or hand function. In both groups, handedness was assessed using the Edinburgh Handedness Inventory, where stroke subjects were asked to evaluate their pre-stroke handedness retrospectively. All subjects gave written informed consent before participating in the experiment. The study was approved by the ETH Ethics Committee EK 2019-N-108 and the Ethics Commission of Baden-Württemberg F-2016-126 and retrospectively registered as a clinical trialFootnote 1.
Robot-assisted assessments
Apparatus
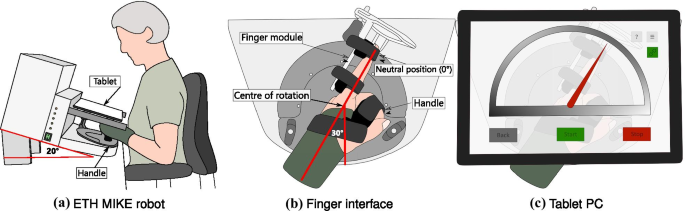
The ETH MIKE (Motor Impairment and Kinesthetic Evaluation)Footnote 2 is a one degree of freedom end-effector robot, which can provide well-controlled stimuli to the index finger and sensitively measure subjects’ kinematic and kinetic responses [41, 42]. The end-effector has its center of rotation aligned with the MCP joint of the index finger. Subjects are seated in front of the device, the hand is placed grasping an easily exchangeable, 3D printed handle, and the index finger is stretched and attached to the end-effector via Velcro straps (Fig. 1a). For a natural and comfortable positioning, the hand of the subject is placed in the device with a
angle from the middle of the end-effector’s workspace (Fig. 1b, c). The device is suitable to test both hands, one hand at a time. A tablet computer with a touch screen is placed directly above the hand, displaying a Graphical User Interface (GUI) programmed in Unity (Unity Technologies, California, USA), that is used as a visual display during the assessment tasks. To minimize cognitive load, the GUI displays a simple gauge with colored indicators for all assessment tasks (Fig. 1c).
from the middle of the device’s workspace), ensuring a comfortable resting position for the wrist. The index finger is attached to an adjustable finger module by Velcro straps. The centre of rotation of the end-effector is aligned with the MCP joint. c A tablet computer is placed above the hand, removing visual cues from the tested hand and providing an interactive graphical user interface displaying a simple gauge with a red indicator
The hardware of the robot consists of one actuator (a DC motor), as well as an incremental encoder, a tachometer and a force sensor. The device is controlled by a real-time embedded board (myRIO, National Instruments, Texas, USA) and programmed in LabVIEW (National Instruments, Texas, USA). The end-effector position, velocity and interaction force signals are recorded at a sampling frequency of 1 kHz. Velocity and force signals are smoothed in real-time using a 1st order Butterworth low-pass filter with 20 Hz cutoff frequency. Post-processing is implemented in MATLAB (Mathworks Inc., Massachusetts, USA).
Robotic assessment battery
The battery of robot-assisted assessments consists of tasks targeting proprioceptive and motor impairments, as well as the interplay between the two (sensorimotor impairments). Specifically, there is one task for proprioception assessment (gauge position matching), three tasks focused on motor impairments (range of motion, maximum force generation and fast target reaching). Additionally, one task is designed to evaluate the ability to integrate proprioceptive information to execute a complex movement, i.e. sensorimotor impairments (trajectory following). All of these tasks are performed on the robotic platform ETH MIKE. A motivation from related literature, the task procedures, and sensor-based metrics extracted from each task are described below.
Gauge position matching task—assessment of proprioceptive impairments: the objective of this assessment is to evaluate the MCP joint proprioception, while minimizing possible confounds coming from motor impairments. The task is based on previous studies that optimized the gauge position matching task procedure [46, 47]. Compared to a 2 alternative forced choice paradigm often used to evaluate somatosensory function [36, 48], the gauge position matching task is faster and does not rely on subjects remembering and comparing positions. The task procedure is the following: after the tested finger has been passively moved to a target angle by the robot, the user is prompted to indicate the perceived finger position on the tablet screen, located directly above the hand, by moving a virtual gauge indicator to a position aligned with the tip of the tested index finger (Fig. 2a). Their view of the hand is constrained by the location of the tablet, hence subjects can not compensate by visual feedback to complete the task. Every trial starts with the robot moving the finger from the neutral position (
angle at the MCP joint) to one of 21 angles (integer values [] in flexion from the neutral MCP joint position) within 3 seconds. In one assessment, each angle is presented once, in a random order. Previous work has shown that sampling each angle once is sufficient to reliably assess proprioception, while minimizing the duration of the test [47]. There is no time constraint for the subjects to indicate the perceived position and no feedback is given about the subject’s performance. To ensure that the task assesses one hand only and does not rely on subject’s ability to indicate the perceived position on the screen with the other hand, the experimenter helps the subject to point to the perceived finger position on the screen. For all stroke and control subjects, the experimenter first asks if the gauge indicator on the tablet screen is below or above the reference position and then moves the gauge indicator slowly in that direction, by dragging it on the touch screen, until the subject says “stop”. Then the experimenter asks for confirmation and allows for final adjustments. For each trial, the absolute error is calculated by taking the absolute value of the difference between the reported and the presented angle. The primary outcome measure is the mean value of this absolute error across all 21 trials, denoted Position Matching Absolute Error. The higher the absolute error, the worse the task performance.
Fast target reaching task—assessment of motor impairments (1): the objective of this task is to quantify subjects’ ability to produce fast ballistic target reaching movements. Target reaching has been used before as an assessment method of motor function deficits [49,50,51,52]. However, in contrast to target reaching tasks typically implemented in literature [49,50,51,52], in the newly proposed task the velocity is of interest and the accuracy of the movement is not considered. We designed the task in a way to minimize the involvement of somatosensory feedback in the movement generation, thereby relying on feedforward control. Subjects are instructed to move as fast as possible, in a single movement, from a starting position to a target, each displayed on the tablet computer screen as a red and green gauge indicator respectively. We therefore expect a ballistic movement, with minimal end-point correction since no visual feedback on the current position is provided and the finger is hidden under the tablet. The movement is performed either in flexion or in extension direction, in a random order. First, the tested finger is passively moved to a starting position by the robot (
from neutral joint angle as starting position for flexion and for extension trials). Then, after a 3-second countdown, subjects are instructed to move as fast as possible to the target (displayed at for flexion and at for extension trials). Four seconds are given for all subjects to move to the target, which was chosen to standardize the protocol and ensure that subjects with a slower reaction have enough time to generate a movement. Subjects are instructed to remain at their position once they believe they have reached the target. One assessment consists of 20 trials (10 times each direction). The primary outcome measure is the mean of the three maximum velocity values (in) over all 10 trials per movement direction (denoted Maximum Velocity Flexion/Extension). The higher the velocity, the better the task performance. Representative velocity profiles are shown in Fig. 3a.
Range of motion task—assessment of motor impairments (2): the purpose of this task is to measure the range of motion of the index finger in flexion and extension direction. The range of motion is regularly evaluated in clinical settings to describe hand impairments [28, 53, 54]. Stroke subjects often show limited range of motion and the ability to extend the finger early post-stroke has even been shown as a predictor of recovery [45]. In this task subjects are instructed to move the index finger (which is secured on the ETH MIKE finger interface) as far as possible first in flexion and then in extension direction. This is repeated three times. Subjects can see the visual feedback of their finger displayed on the tablet computer. Afterwards, the same task is repeated in a passive manner, meaning that the experimenter moves the subject’s finger in flexion (until the end of the range of motion of the robot or until the subject says “stop” due to discomfort) and then in extension (until the experimenter detects tension in subject’s finger by feeling some resistance against the movement or until the subject says “stop”), while the subject is instructed to relax his/her finger. Here, the tablet computer is removed so as to not obstruct the experimenter that induced the motion. For each repetition, the difference between the maximum position in flexion and the maximum position in extension (measured in degrees) is calculated (denoted as Active/Passive Range of Motion – AROM/PROM). The primary outcome measure is the mean value across three repetitions for both AROM and PROM. The higher the ROM, the better the task performance. Representative position profiles are shown in Additional file 1: Fig. SM1a.
Maximum fingertip force generation task—assessment of motor impairments (3): the objective of this task is to measure maximum fingertip force. Assessments of grip strength are often performed in clinical settings in patients after stroke [55, 56], as weakness is frequently present after stroke and is linked to the damage to the corticospinal tract [13]. In this task procedure the end-effector is first blocked by a fixation mechanism, located at a
flexion angle at the MCP joint (with respect to a neutral position where all phalanges are aligned). The subjects are instructed to generate maximal force with their index finger for an indicated period of time (3 s), preceded by a 3 s preparation phase. No verbal or visual feedback related to the magnitude of the generated force is provided to the participants during the task. Three repetitions are performed first in flexion and then in extension direction. The primary task metric is the mean of the maximum force over three trials for both the flexion and extension direction, measured in Newtons by the force sensor located at the end-effector (denoted Maximum Force Flexion/Extension). The higher the force, the better the task performance. Representative force profiles are shown in Additional file 1: Fig. SM2a.
Trajectory following task—assessment of sensorimotor impairments: the aim of this task is to assess finger dexterity, which relies both on proprioceptive function and motor execution. Trajectory following has been used previously to evaluate fine motor control [57,58,59]. First, the index finger is passively moved to a starting position by the robot (
flexion angle at the MCP joint). After a three second countdown, a trajectory is displayed on the tablet screen in the form of a moving gauge indicator, which the subjects are instructed to follow as accurately as possible. The vision of the actual finger position is not displayed on the screen, to ensure that subjects rely on proprioception to guide the motion. Two trajectory scenarios are displayed (slow and fast) in order to diversify the task. Each trajectory consists of three superimposed sine waves, each of different frequency and the same amplitude (). The slow trajectory consists of the following sine wave frequencies: 0.03 Hz, 0.07 Hz and 0.13 Hz, while the fast trajectory is composed of 0.10 Hz, 0.20 Hz and 0.40 Hz. One trial lasts 30 seconds and in total there are six trials in one assessment (three times each trajectory, first 3 times slow, then 3 times fast). For each trial, the tracking error between the trajectory displayed on the screen and the performed motion is calculated (Root Mean Squared Error RMSE [57]). The primary outcome measure is the mean across the three trials for the slow and the fast trajectory (denoted Tracking Error RMSE Slow/Fast). The higher the tracking error, the worse the task performance. Representative trajectories are shown in Fig. 4a.
Clinical assessments
The following clinical assessments were performed by a trained physiotherapist as a part of the study protocol. The kinesthetic Up-Down Test (kUDT) as part of the Nottingham Sensory Assessment (NSA) was chosen as a measure of proprioception (performed with the forearm fully pronated and the wrist in a neutral position) [60]. In order to keep the scoring system of the kUDT from the NSA consistent with the commonly used Erasmus modified Nottingham Sensory Assessment [61], scores 1 and 2 were grouped together as score 1 and the best score was assigned the value 2. To clinically evaluate motor impairments, the Fugl-Meyer Upper Limb Motor Assessment (FMA) was used [23]. The Box & Block Test of Manual Dexterity (BBT) was selected as an assessment of combined sensorimotor function and activity limitations [27] and it was completed for both hands. To quantify cognitive function, the Montreal Cognitive Assessment (MoCA) was performed [62]. Finally, the Modified Ashworth Scale (MAS), performed at the MCP joint of the index finger, was used as a measure of spasticity [63].
Experimental protocol
Two testing sessions on two separate days were conducted by the same experimenter to evaluate test-retest reliability of robotic task metrics in stroke subjects. Clinical assessments were performed in a separate session. For the control subjects, the protocol consisted of only one experimental session with the robot.
Subjects were seated in front of the robotic device and the height of the chair and the armrests was adjusted to a comfortable seating position close to the robot (Fig. 1a). A wrist splint was used to ensure that the MCP joint was tested in isolation without any compensatory movements from the wrist. The elbow of the subjects was placed on the cushioned armrest and subjects were instructed to keep it close to their body and to avoid compensatory movements throughout the trial. The hand was strapped to the handle after ensuring optimal alignment of the forearm and the wrist joint with the orientation of the handle (neutral position of the wrist,
from the middle of the device workspace—Fig. 1b). The index finger was attached to the finger module. The robotic assessments were always started with the range of motion and maximum fingertip force generation tasks, as they were the least complex and helped subjects to get familiar with the device. The order of the other three tasks, as well as the starting hand were randomized. Afterwards, subjects performed the assessments in the same order with the other hand. There was a familiarization round before each task. It consisted of a shortened version of the task, with only half the number of trials, and where subjects were instructed and encouraged to ask any questions they may have related to the task.
More at link.
No comments:
Post a Comment